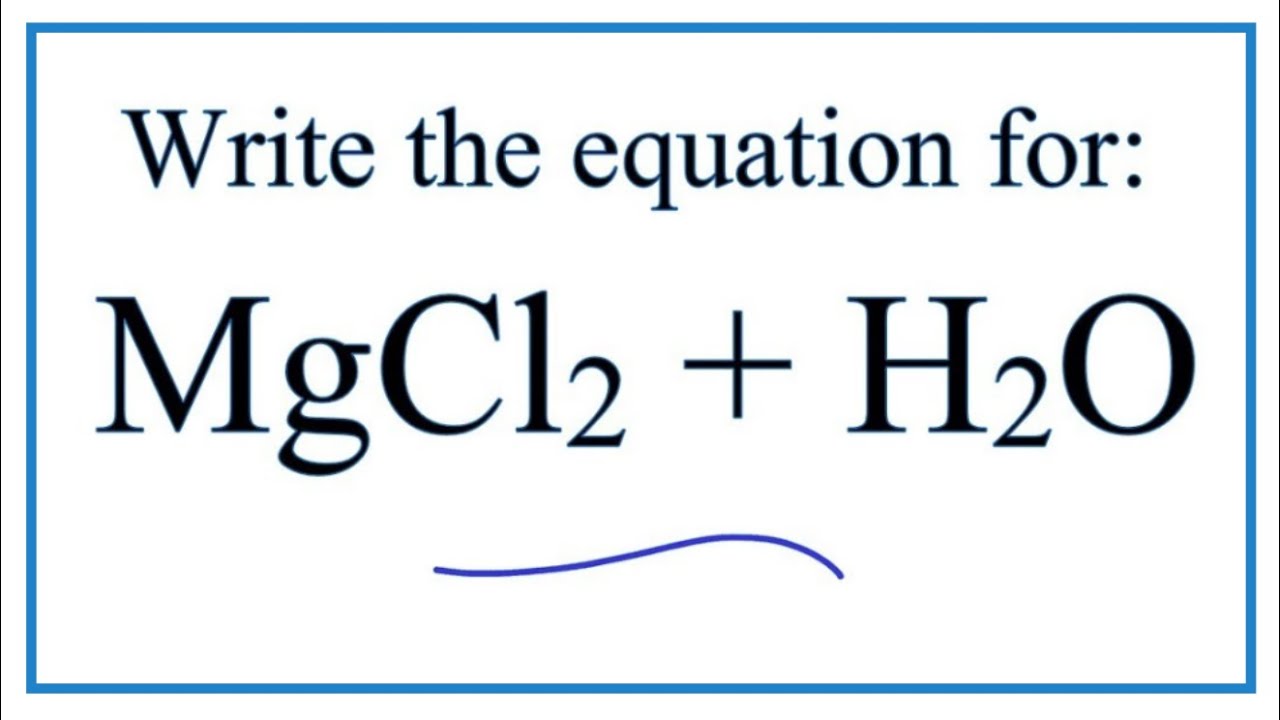Ideal Reaction Of Magnesium Chloride With Water

What type of intermolecular force will act in Magnesium chloride MgCl2 dissolved in water H2O.
Reaction of magnesium chloride with water. When magnesium ions are solvated from the solid lattice there is enough attraction between the 2 ions and the water molecules to form coordinate dative covalent bonds between the magnesium ions and lone pairs on surrounding water molecules. As an approximation the simple ionic chlorides sodium and magnesium chloride just dissolve in water. The products are a salt MgCl2 and and water.
NaCl and MgCl2 are ionic and water soluble. You could also use 112 ratio but this ratio is very mild. Electrolysis An insoluble magnesium hydroxide precipitates to the bottom of a settling tank whence it is pumped as a slurry filtered converted to magnesium chloride by reaction with hydrochloric acid and dried in a series of evaporation steps to 25 percent water content.
The reaction of magnesium oxide with water. If I want to make a Magnesium oil spray to use topically what ratio of water to Magnesium Chloride should I use. The other chlorides all react with water in a variety of ways described below for each individual chloride.
Hence any reaction of a chloride with water depends upon the element attached to the chloride. The solid magnesium hydroxide from the initial reaction is then reacted with hydrochloric acid to produce magnesium chloride. The reaction with water is known as hydrolysis.
Magnesium oxide reacts with water forming magnesium hydroxide. An inorganic compound consisting of one magnesium and two chloride ions. Mg s H 2 O g MgO aq H 2 g Mg s 2 H 2 O g Mg OH 2 aq H 2 g.
The reaction of magnesium oxide with carbon dioxide carbonic acid gas. One-half cup or 4 ounces of Magnesium Water yields 90 mg of magnesium. Magnesium chloride react with water MgCl 2 H 2 O MgO 2HCl Check the balance Magnesium chloride react with water to produce magnesium oxide and hydrogen chloride.