Formidable Balanced Equation For Complete Combustion Of Propane
Complete combustion of propane produces about 50 MJkg of heat.
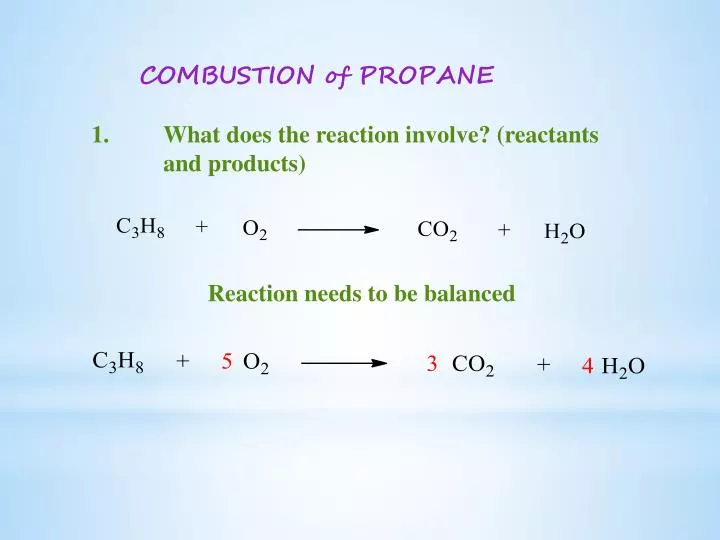
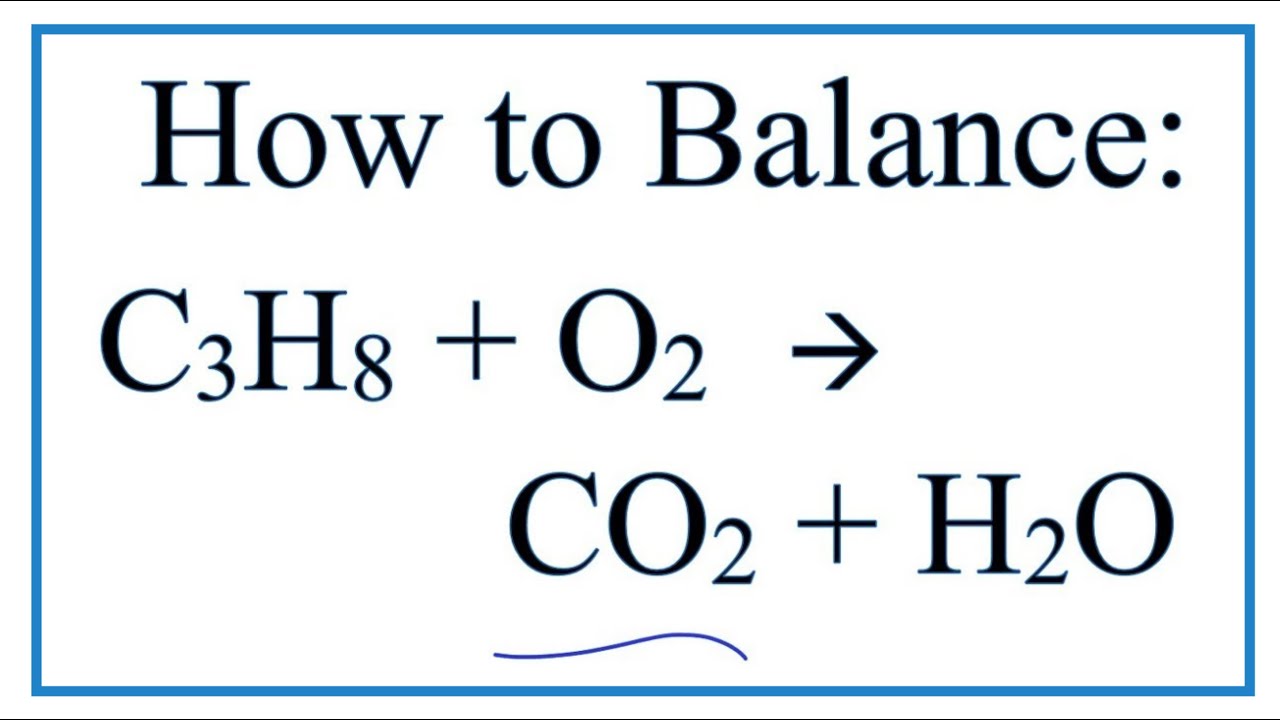
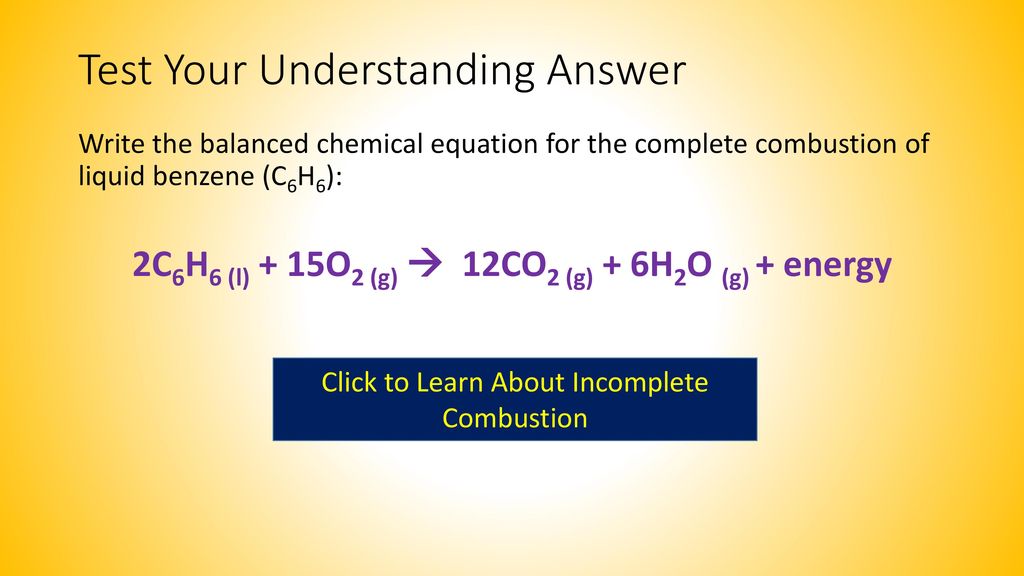
Balanced equation for complete combustion of propane. The propane equation for complete combustion of propane involves propane and oxygen as fuel input and carbon dioxide water heat and possible carbon monoxide as the outputs. Propane oxygen carbon dioxide water C3H8. A 1200g H2O are produced.
C 300 mol CO2 are produced. By signing up youll get thousands of step-by-step solutions to your. The complete combustion of propane C3H8 g is represented by the equation.
C3H8g5O2g3CO2g4H2Og Which statement is correct about the complete combustion of 300 mole of propaneC3H8. Complete combustion does NOT give carbon monoxide or sootCheck me out. C3H8 g 5 O2 g 3 CO2 g 4 H2O l ΔH -2220 kJ How much heat is evolved in the complete combustion of 200 L C3H8 g at STP.
Best Answer This is the best answer based on feedback and ratings. There will be 10 oxygens on the products side and 2 on the reactants so to balance these out we multiply the 02 on the reactants side by 5. Complete combustion does NOT give carbon monoxid.
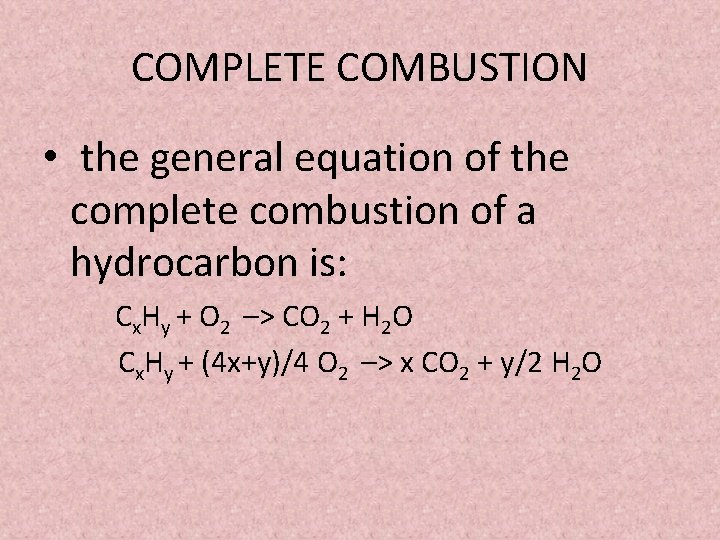
With the states of matter describing the complete combustion of propane gas C3H8. D 1200 mol H2O are produced. Fuel O2 -CO2 H2O The coefficients of the balanced equation will change depending on the fuel.
C3H8g 5O2g 3CO2g 4H2Og But if there is not enough oxygen available to give the complete combustion then there will be a mix of solid carbon soot carbon monoxide gas and carbon dioxide gas. 1- Firstly the carbon in the hydrocarbon propane oxidizes with oxygen to produce carbon dioxide. Download Organic Chemistry Mindmap Notes Organic compounds Compounds from Living Things compounds found in living organisms Examples sugar fats plant oils.