Simple Rusting Of Iron Ppt

The rusting of iron can lead to damage to automobiles railings grills and many other iron structures.
Rusting of iron ppt. The most common method is hot-dip galvanizing in which parts are submerged in a bath of molten zinc. Mixing strong acids and water. F The iron construction structure is tied with a bag filled with magnesium or zinc powder or magnesium or zinc pieces to protect the iron from getting corroded.
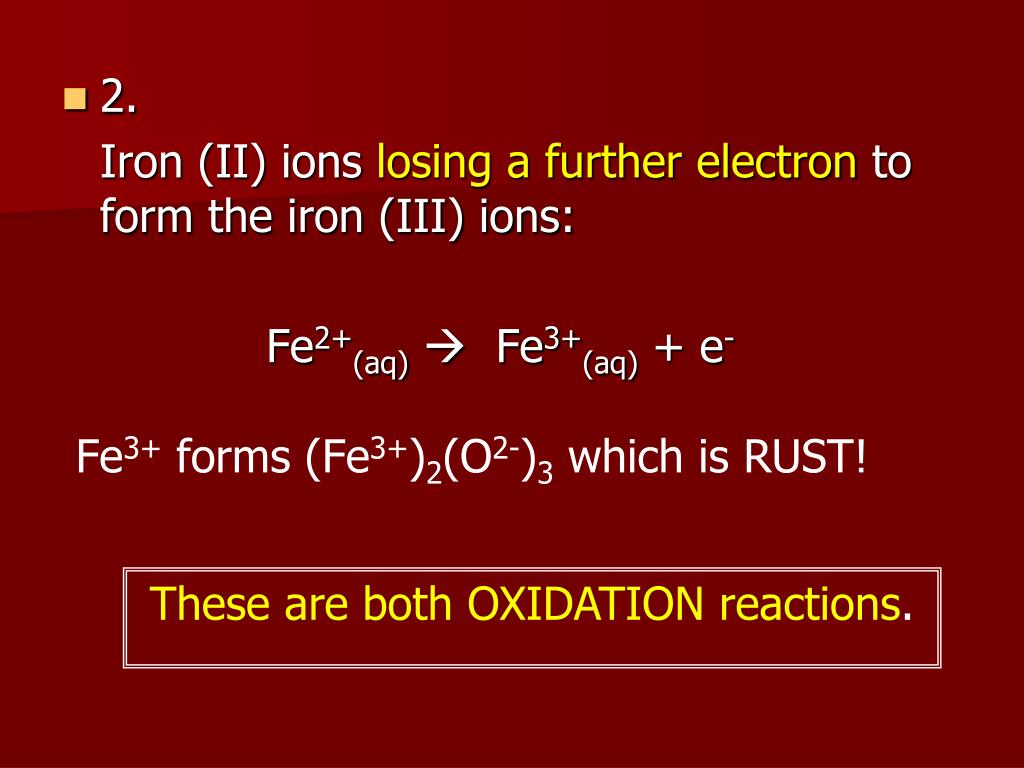
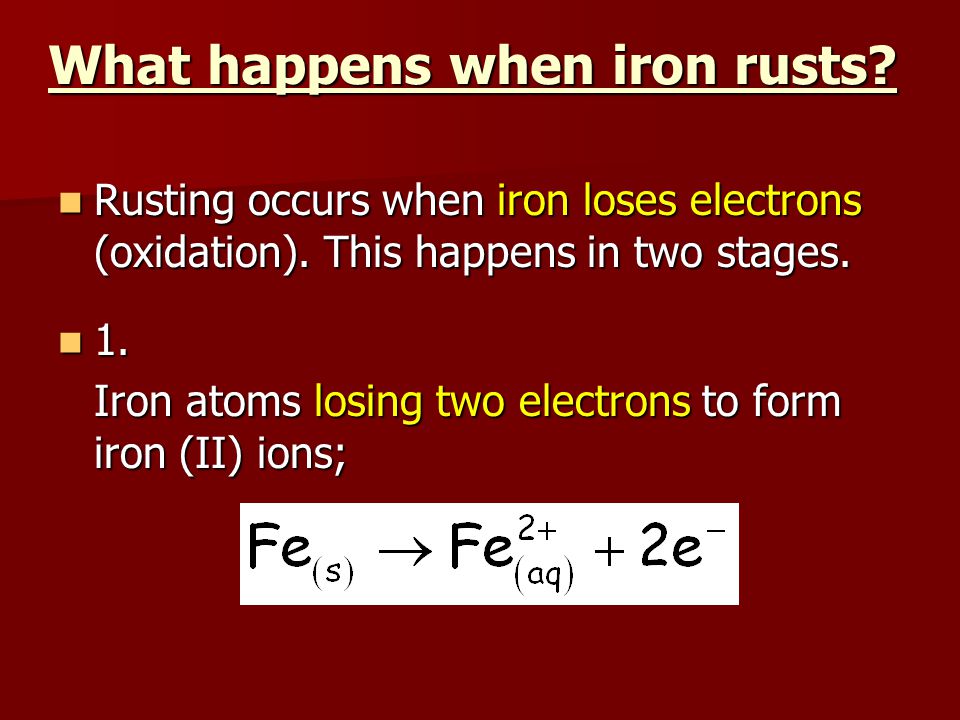
Four equation for rusting. Both oxygen from the air and water are required for rusting. Combining atoms to make a gas molecule.
It is very-very slow process. Combining atoms to make a gas molecule. Hydrated iron III oxide 4.
In the presence of moist air containing dissolved oxygen or carbon dioxide the commercial iron behave as if composed of small electrical cells. Combining Atoms To Make PPT. Describe the equation involve in rusting process Describe the conditions in which rusting occurs.
Chemically rust is a hydrated ferric oxide Titanics bow exhibiting microbial corrosion damage in the form of rusticles Rusting an Electrochemical Mechanism. Rusting of IronWhen a piece of iron is left out in the open for a while a film of brownish substance gets deposited on its surface called rustRusting occu. PowerPoint PPT presentation free to view.
This website and its content is subject to our Terms and Conditions. Rusting results the iron object becoming weaker. Tarnishing of silver wares in H2S laden air due to formation of silver 2.