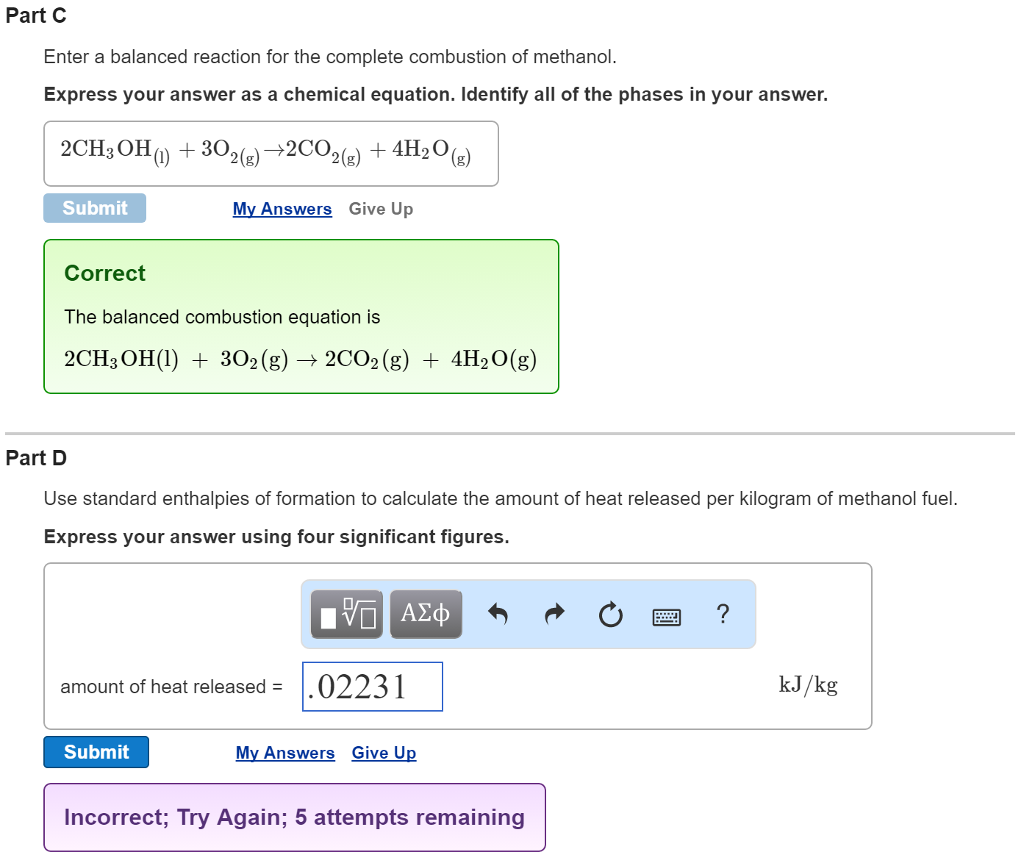
Exemplary Methanol Complete Combustion Balanced Equation

2CH3OHl 3O2g 2CO2g 4H2Ol.
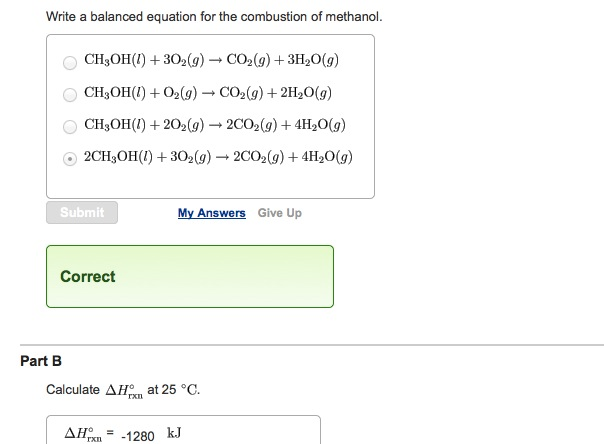
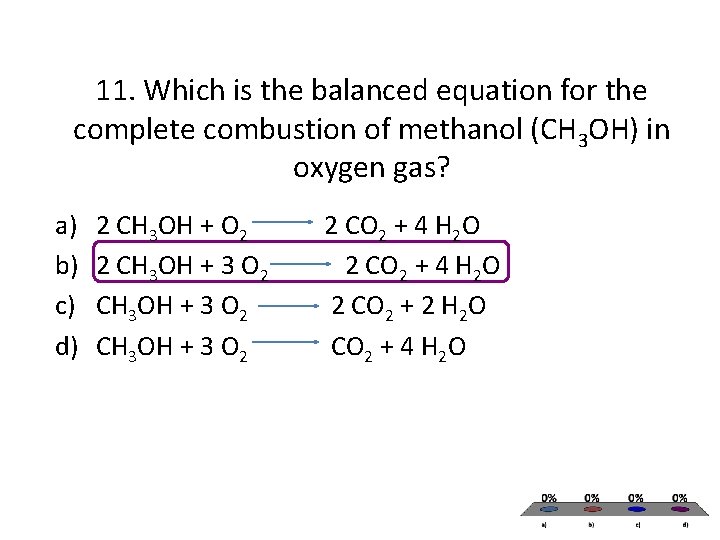
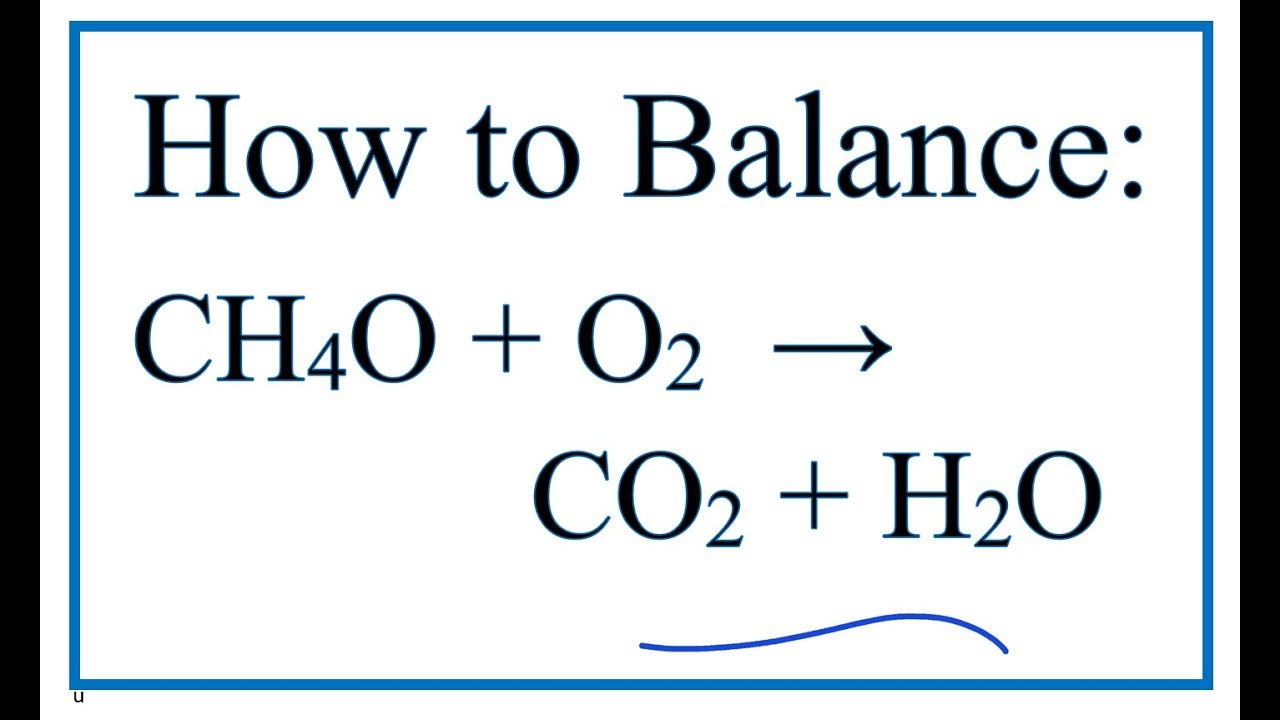
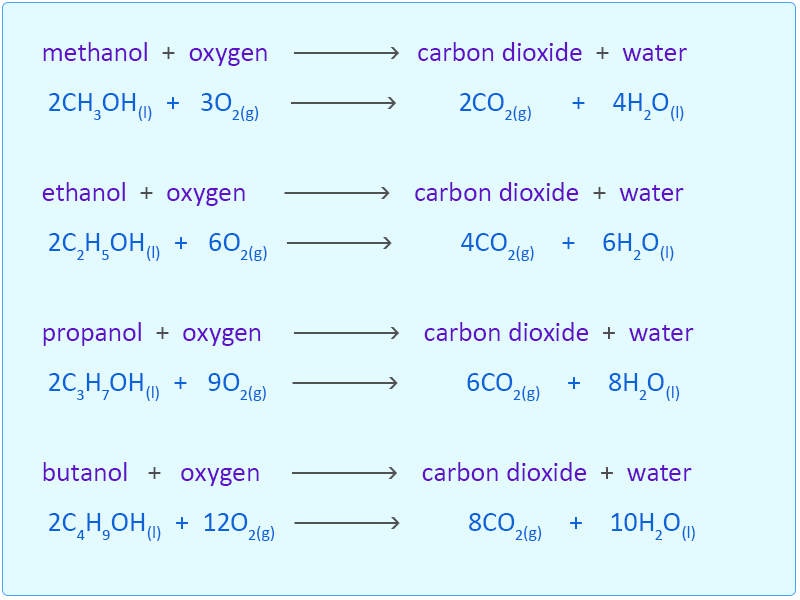
Methanol complete combustion balanced equation. 2CO2 4H2O is the balanced equation for the combustion of methanol. CH3OH l 3O2 g rightarrow CO2 g 3H2O g CH3OH l O2 g rightarrow CO2 g 2H20 g CH3OH l 2O2 g rightarrow 2CO2 g 4H20 g 2CH3OH l 3O2 g rightarrow 2CO2 g 4H20 g Correct Calculate Delta H degree rxn at 25 degree C. Thus if two moles of CH4 undergo complete combustion four moles of.
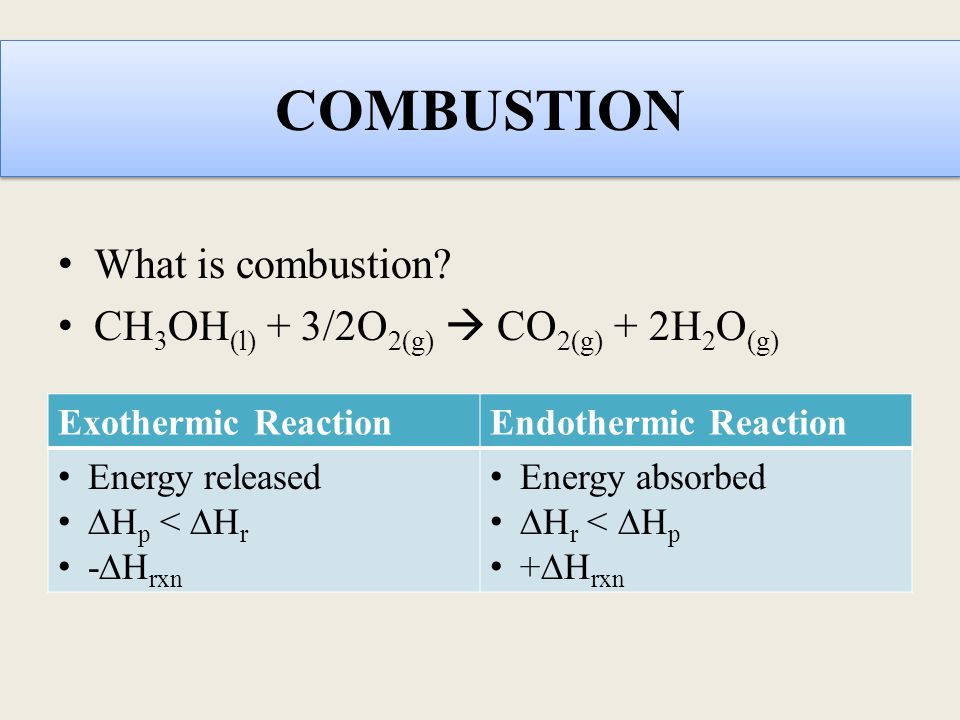
The combustion of other alcohols are given below. CH4 g 2 O2 g CO2 g 2 H2O g Which of the following statements concerning this chemical equation isare correct. It says that you get twice as many moles of water as you get carbon dioxide.
325 mol x 2. For methanol the standard molar enthalpy of complete combustion in an open system is calculated to be kJmol. Use the lowest possible coefficients.
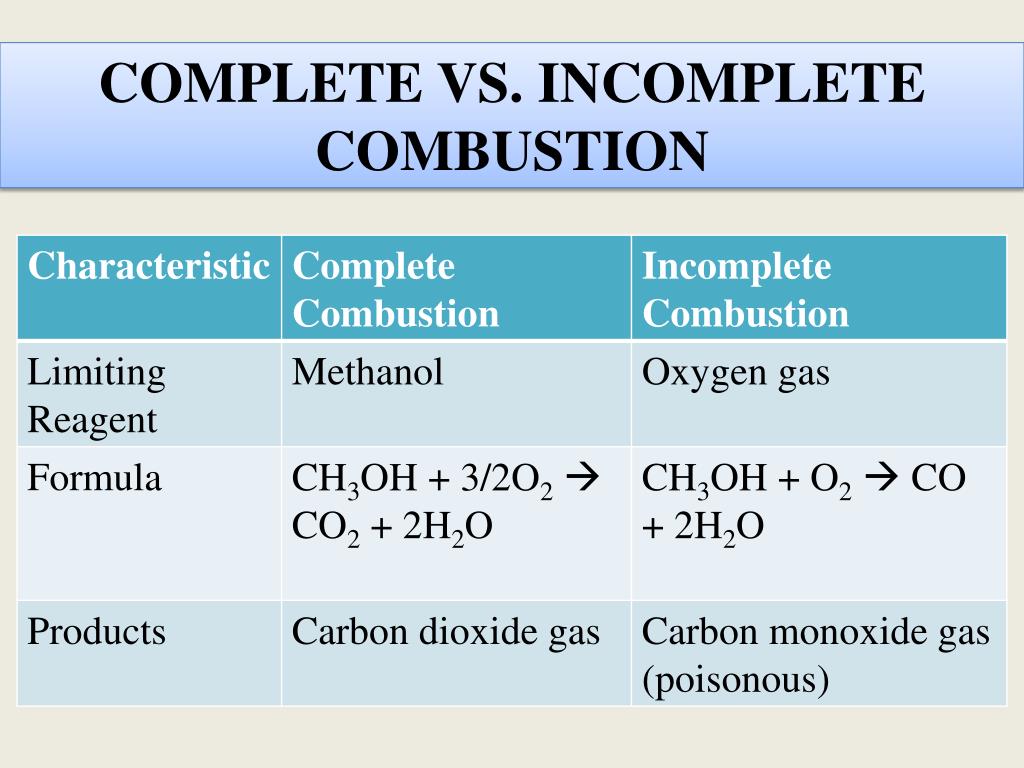
2CnH 2n1OH l 3nO2g 2nCO2g 2n 2H 2Ol n 123. Which is confusing me. The incomplete combustion of a hydrocarbon usually produces a sooty flame due to the presence of carbon C or soot as a product of the incomplete combustion.
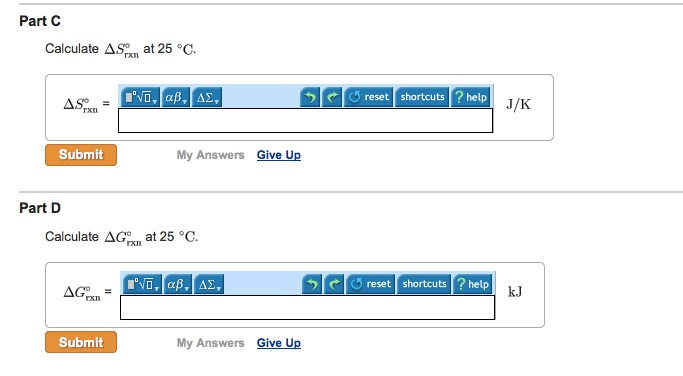
Delta H degree rxn -1280 kJ Calculate Delta S degree rxn at 25 degree C. Type your answer using the format CO2 for CO2. 2CH3OH 3O2 --.
Meave60 Media Owl Aug 22 2014 The balanced chemical equation for the combustion of liquid methanol in oxygen gas to yield carbon dioxide gas and liquid water is. 2 C H 3 O H l 3 O 2. Write a balanced equation for the combustion of methanol.