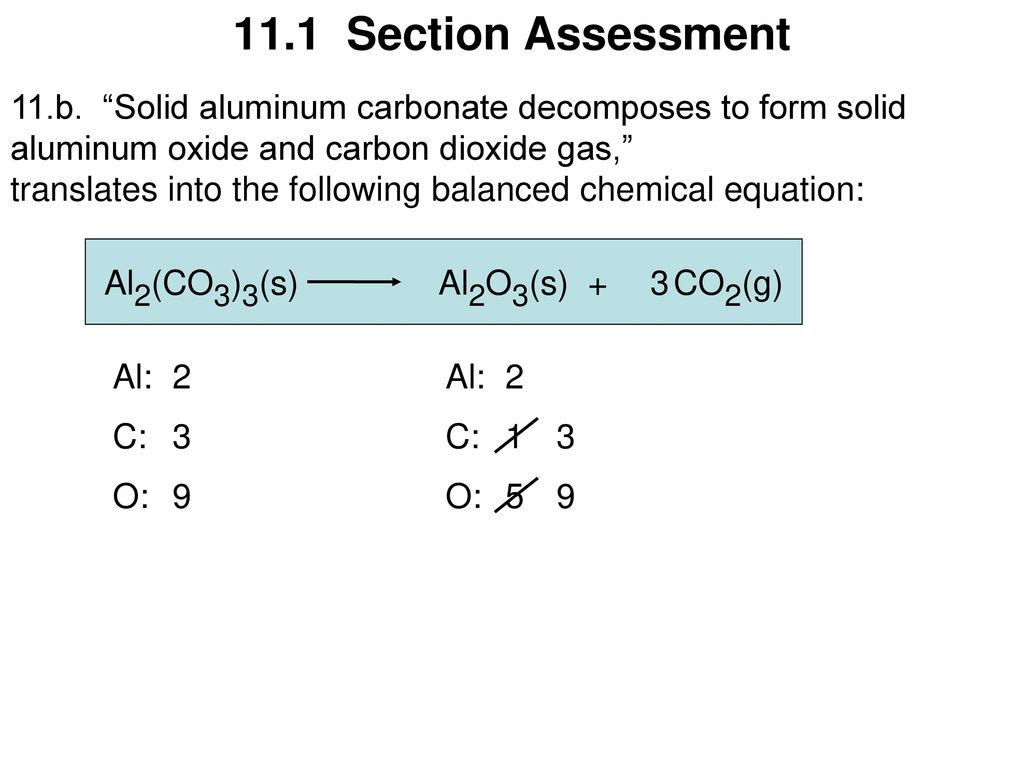
Fantastic How Is A Skeleton Equation Different From A Balanced Equation

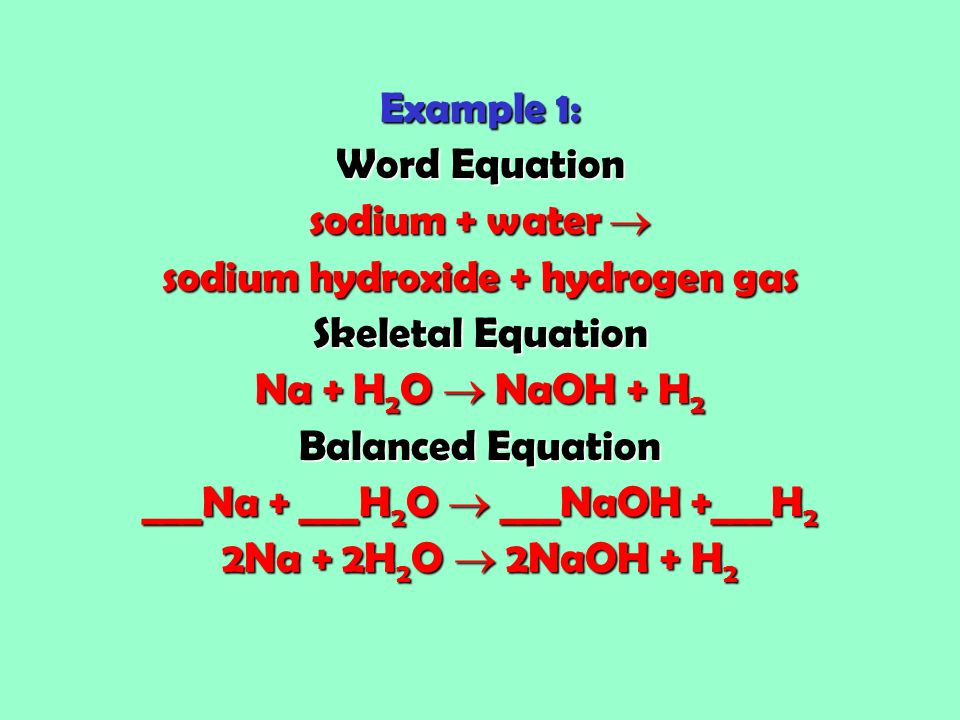
In a skeleton equation you put chemical formulas in place of chemical names.
How is a skeleton equation different from a balanced equation. Describing Chemical Reactions What is a skeleton equation. What is skeleton reaction. Mg s Br 2 g -- MgBr 2 aq answer choices.
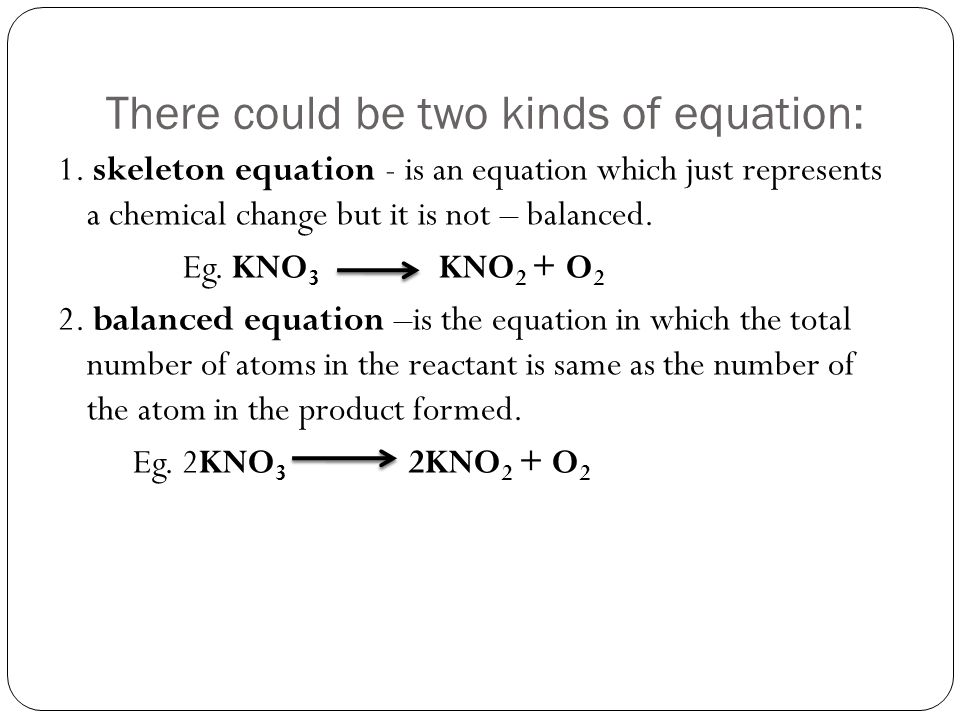
OPO POO FeOH - Foo HO Naci HSO Na HCI. Unbalanced chemical equation is called skeletal chemical equation Law ofconservation of mass -States that mass can neither be created nor be destroyed. Can a skeleton equation equal a balanced equation.
The key difference between balanced equation and skeleton equation is that balanced equation gives the actual number of molecules of each reactant and product involved in the chemical reaction whereas skeleton equation gives only the reactants of the reaction. Methane oxygen water carbon dioxide Skeleton equation. What is a catalys.
The key difference between balanced equation and skeleton equation is that balanced equation gives the actual number of molecules of each reactant and product involved in the chemical reaction whereas skeleton equation gives only the reactants of the reaction. Ionic charges are not yet supported and will be ignored. Differentiate Between A Balanced And A Skeletal Chemical Equation Differentiate Between a Balanced and a Skeletal Chemical Equation In a balanced chemical reaction the number of atoms in each element of a reactant side should be equal to the number of atoms of that element on the product side.
Is the following equation word or skeleton. A balanced chemical equation occurs when the number of the atoms involved in the reactants side is equal to the number of atoms in the products side. How is a balanced chemical equation different from a skeleton equation.
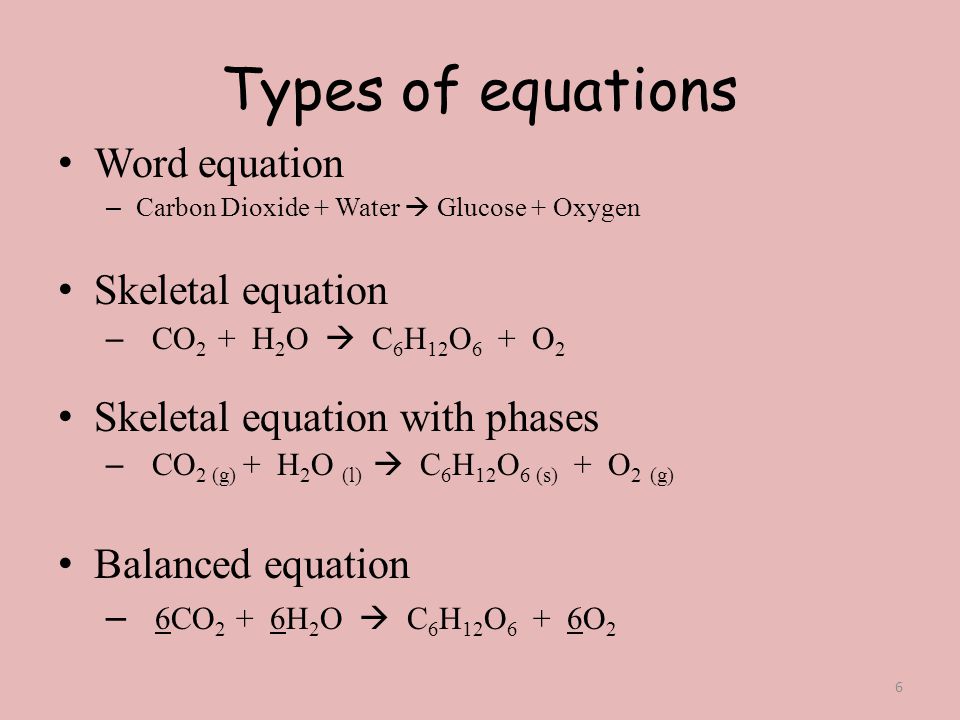
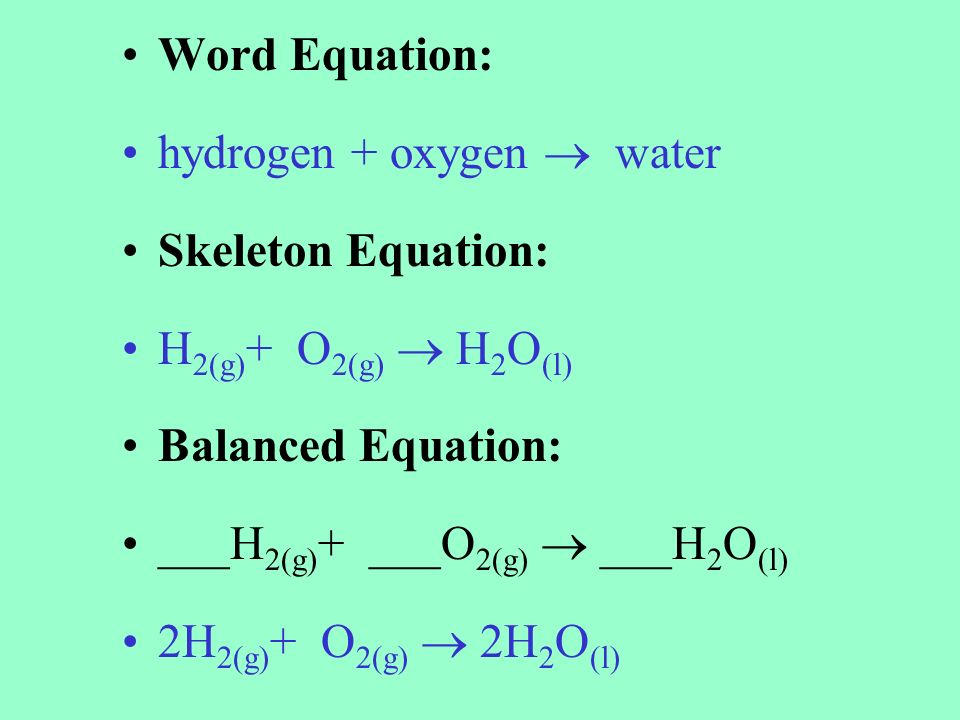
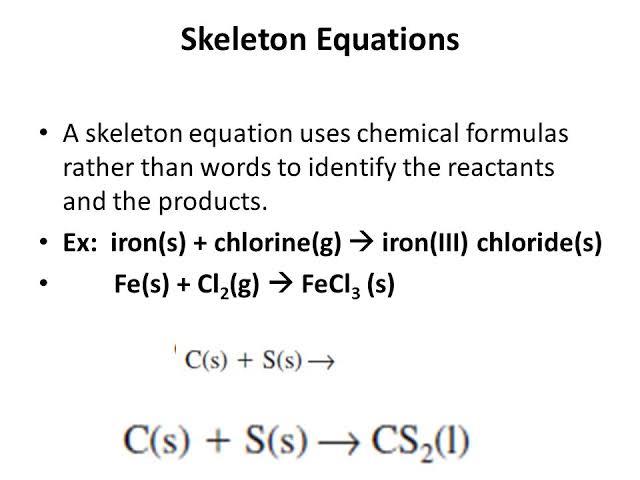
Skeletal chemical equation is a representation. The skeleton equation is the intermediate step which enables the conversion of an equation expressed in words to an actual balanced chemical equation. Balance the following equations.

.PNG)




.PNG)