Matchless Combustion Of Methane Equation With States

Complete combustion does NOT give carbon monoxide or sootCheck me out.
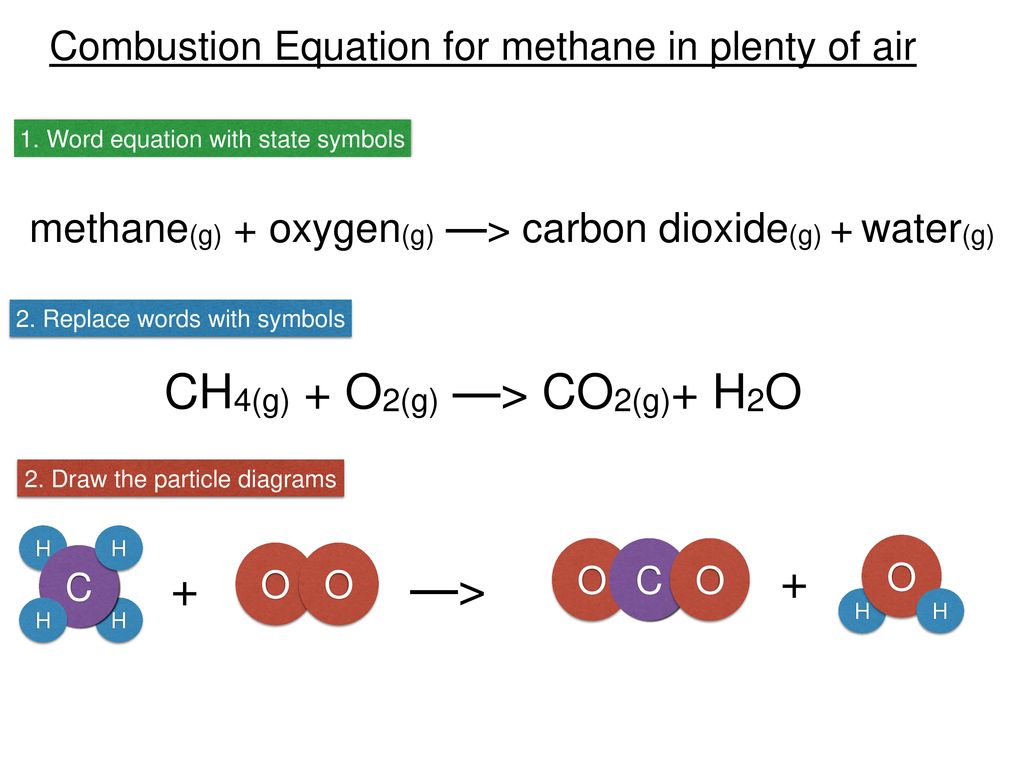
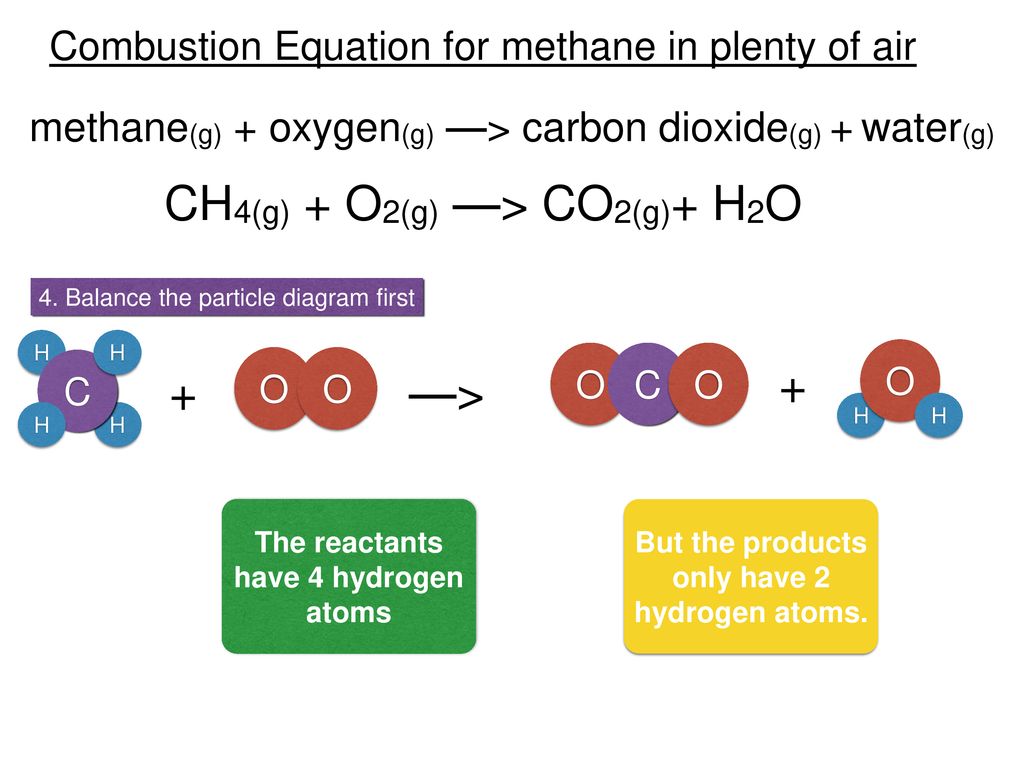
Combustion of methane equation with states. Fuel O 2- CO 2 H 2 O. CH4 g 2 O2 g CO2 g 2 H2O g Which of the following statements concerning this chemical equation isare correct. CH4 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O.
The most common are single replacement and combustion reactions. Combustion of methane Methane is a natural gas that is extracted and used as a fuel. A methane molecule is composed of one Carbon atom and 4 Hydrogen atoms.
Combustion of fuel results from the equation of stoichiometry of oxygenfuel reaction. Stoichiometric air means the minimum air in stoichiometric mixture. First determine the moles of methane.
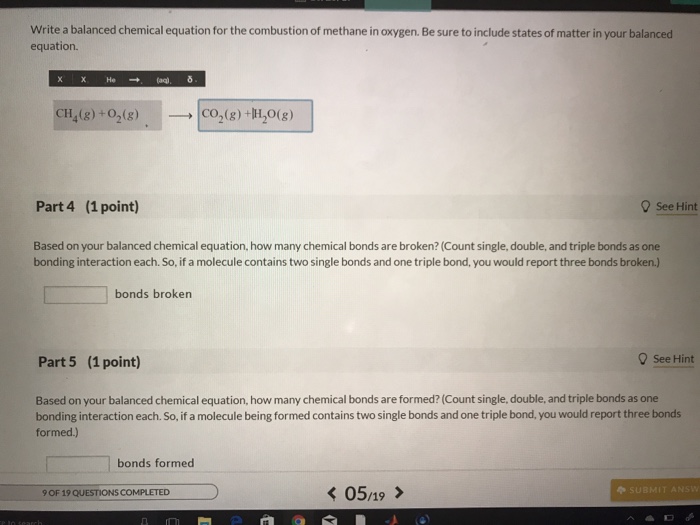
Count single double and triple bonds as one bonding. The equation is balanced because the number of atoms for every element is the same on both the reactant and the product sides. Write a balanced chemical equation for the combustion of methane in oxygen.
CH4 O2 ----- CO2 CO H2O Ill leave the equation unbalanced for you to balance as. We use a set of rules to assign oxidation numbers or states to each of the atoms in the reaction. Were asked to give the answer to the nearest kilojoule per mole.
The classification of combustion phenomena into premixed and non-premixed combustion is used throughout this text. Quick summary of the word and chemical equation for the complete combustion of Methane. Here are the equations that model its complete combustion.