Stunning Reaction Between Ammonia And Hydrogen Chloride
A combination of ammonia and hydrogen peroxide is often used in the process of bleaching hair.
Reaction between ammonia and hydrogen chloride. An exothermic reaction takes place between ammonia and neutral gas. In water the reaction between ammonia NH3 and hydrogen chloride HCl is a textbook example of acid-base chemistry most of us learn in. This results in the hair becoming lighter.
Hydrogen peroxide has the necessary properties to remove the color from hair - it oxidizes one of hairs melanin pigments to a colorless substance. Energy Change in Chemical Reactions. Anhydrous magnesium chloride has been obtained as a white powdered material with a purity of 995999 per cent as calculated by the ClMg ratio.
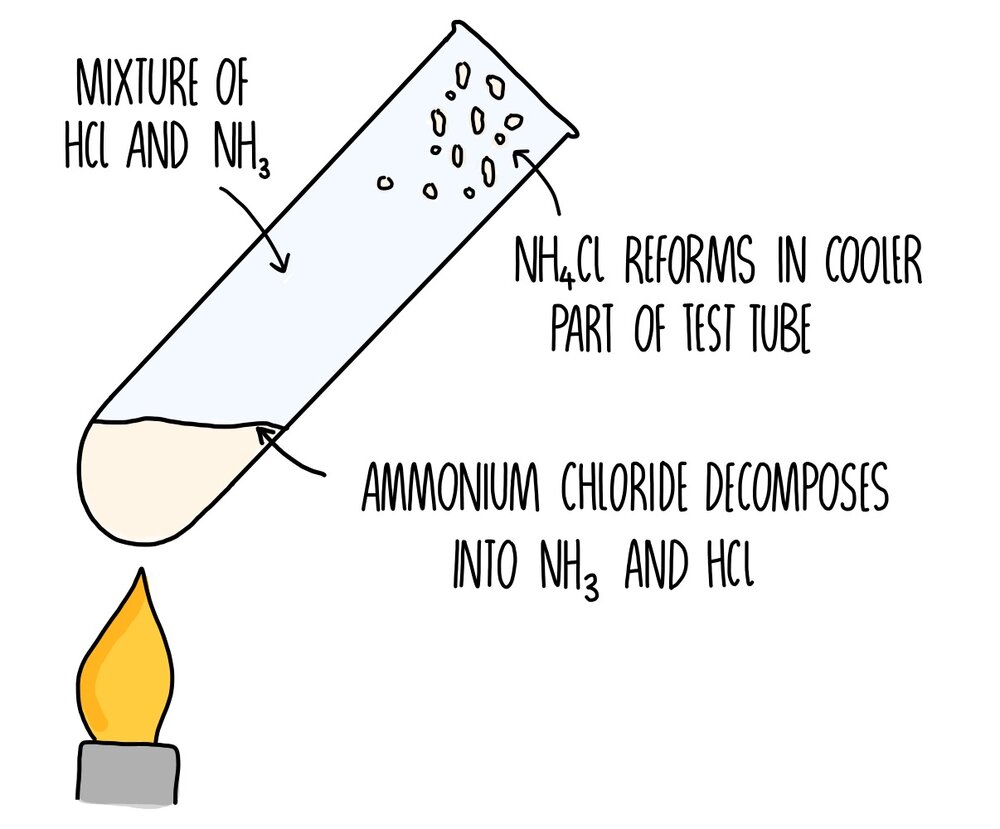
The hydrogen is removed by the chloride ion. When ammonia reacts with hydrogen chloride gas it produces white fumes of ammonium chloride. Ammonia solution is a solution of ammonia gas in water.
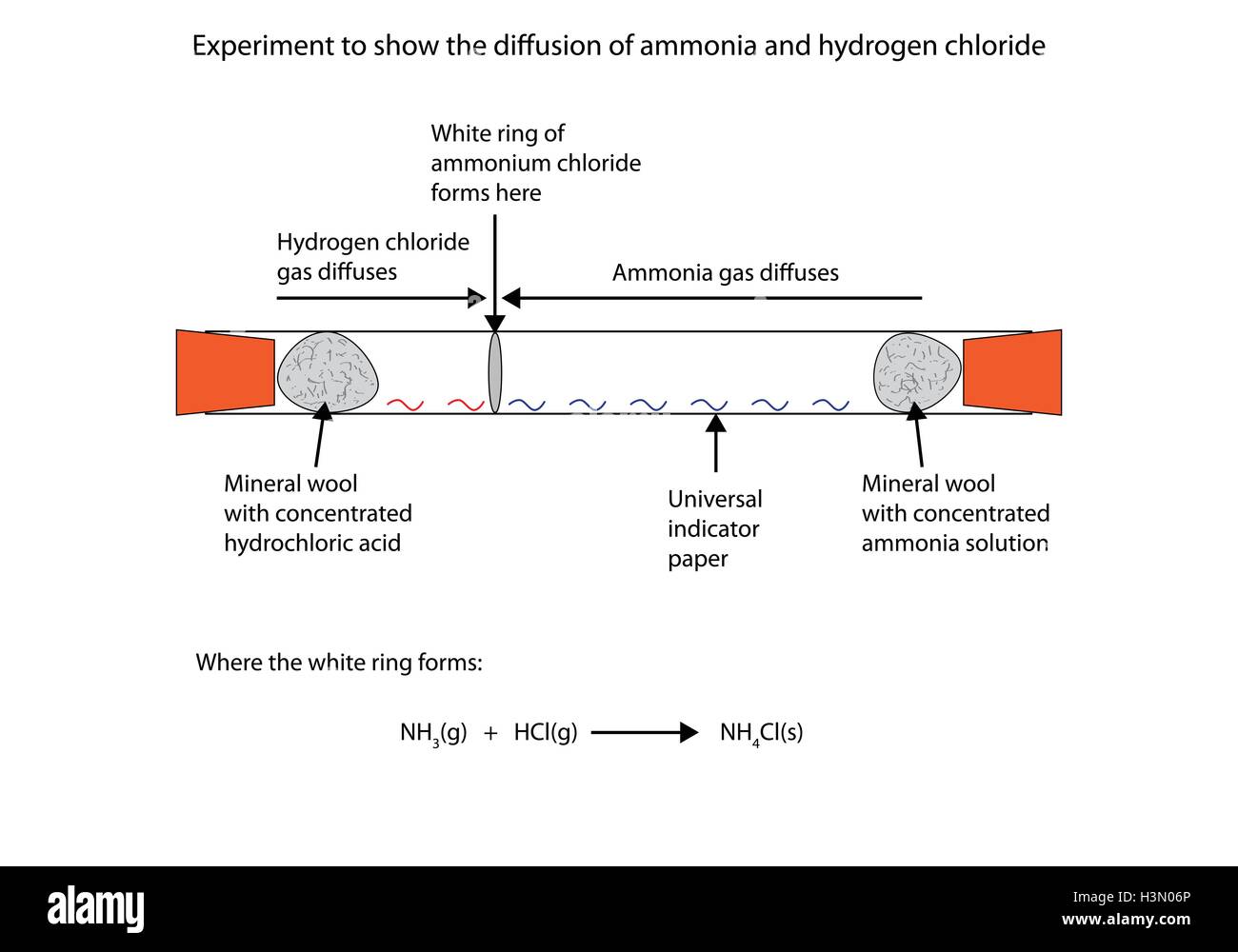
Diffusion of Gases Ammonia and Hydrogen Chloride In this classic demonstration from the Royal Society of Chemistry cotton wool soaked in ammonia and hydrochloric acid are placed at either end of a sealed tube. Notice that unlike the reactions between ethanoyl chloride and water or ethanol hydrogen chloride isnt produced - at least not in any quantity. Any hydrogen chloride formed would immediately react with excess ammonia to give ammonium chloride.
Concentrated solutions give off hydrogen chloride and ammonia gases. The reaction between ammonia and hydrogen chloride If these colourless gases are allowed to mix a thick white smoke of solid ammonium chloride is formed. The procedure involves the intermittent reaction between sublimed magnesium chips and anhydrous hydrogen.
The hydrogen chloride produced would at once react with any excess ammonia present to form ammonium chloride. It also shows that ammonia and hydrogen chloride. A white solid product is formed which is a mixture of ethanamide an amide and ammonium chloride.