Divine Balanced Chemical Equation Hcl And Naoh

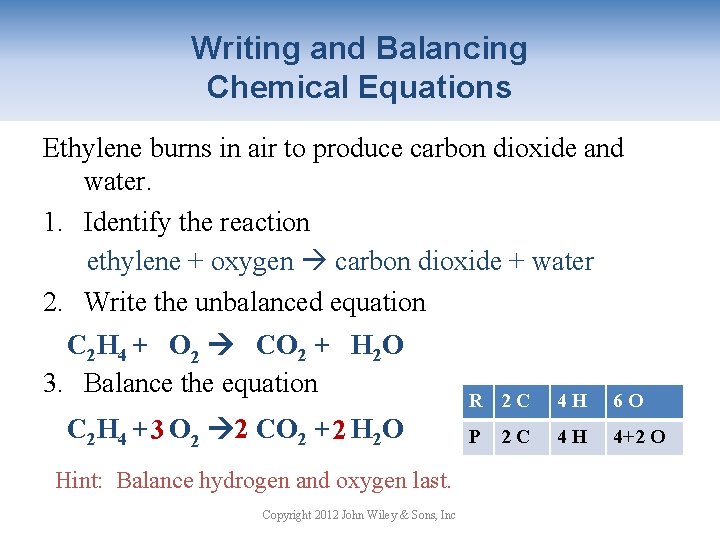
H₂SO₄ NaOH -----Na₂SO₄ H₂O Step-2In the left side we have H SO₄ Na O To balance this reaction means we need to equalize the number of these above atoms and polyatomic ion.
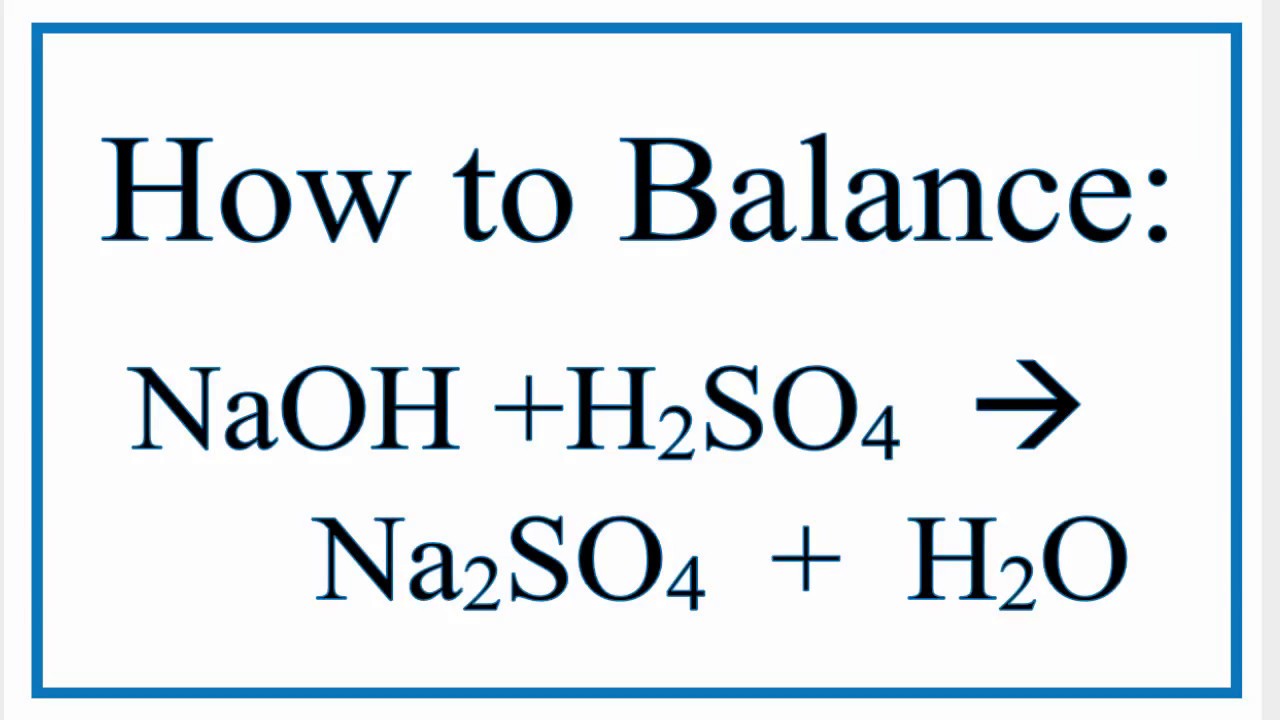
Balanced chemical equation hcl and naoh. If you know that titrating 5000 ml of an HCl solution requires 2500 ml of 100 M NaOH you can calculate the concentration of. This reaction is classified as an exothermic reaction. Sodium Hydroxide and Sulfuric Acid Balanced Equation Now I will balance sulfuric acid and sodium hydroxide reaction.
Heres our balanced equation. The answer will appear below. First we balance the molecula.
Ionic charges are not yet supported and will be ignored. Instructions on balancing chemical equations. We do this by first expressing the chemical formulas of each substance as.
2 HClaq CaCO 3 s ----- CaCl 2 aq H 2 Ol CO 2 g The mixture was then titrated with 0111 M NaOH. For the balanced chemical equation HCl NaOH H2O NaCl if 300 moles of HCl react with enough NaOH how much water will be formed. You can see from the equation there is a 11 molar ratio between HCl and NaOH.
How do you calculate the enthalpy change of HCl and NaOH. Strong acids and strong bases are considered strong electrolytes and will dissociate completely. NaOHaq HClaq NaClaq H2Ol.
NaOH HCl NaCl H2O. Become a member and unlock all. What mass of NaOH was in the sample.