Nice Write An Example Of Neutralization Reaction
We will show two examples of a titration.
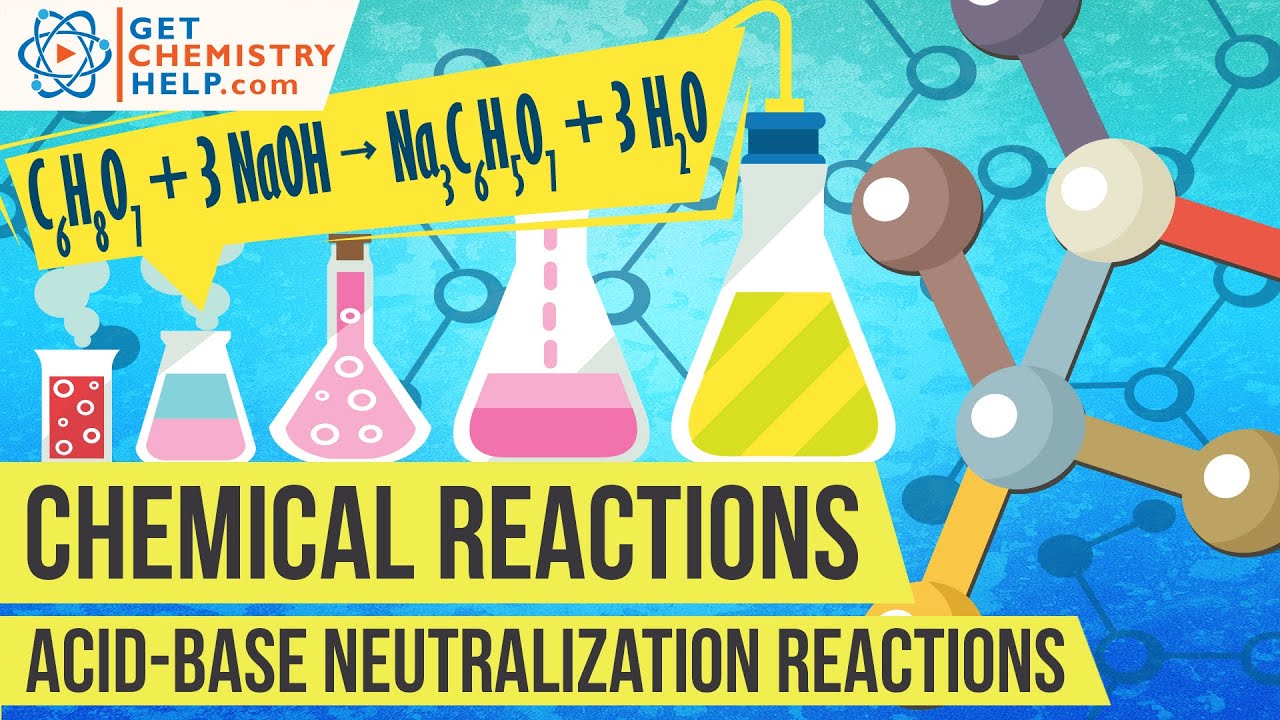
Write an example of neutralization reaction. Lets consider a few neutralization reactions and how we write the equations. When we write neutralization equations we generally do not show hydronium or hydroxide ions and we generally show ionic species as distinct compounds. Consider the reaction between hydrochloric acid and sodium hydroxide.
Neutralization Reaction Acid-Base Reaction to form Salt and Water Relation Between the. I point out that a neutralization reaction produces a salt and water and so if you can write the salt you can write the reaction. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase.
A neutralization reaction is when an acid and a base react to form water and salt and involves the combination of hydrogen ions and hydroxyl ions to generate water. One of the most common and widely used ways to complete a neutralization reaction is through titration. Neutralization reactions are also commonly used in industry as well.
When soil is acidic plant growth is stymied or if it is acidic enough stopped altogether. This is an example of a neutralization reaction. Have you ever been.
It is called neutralization because the acids have a low pH lower than 7 and the bases have a high pH higher than 7. Neutralization is a type of chemical reaction in which a strong acid and strong base react with each other to form water and salt. In order to retrieve the pain we take bases such as milk of.
The essential reaction of chemical leavening agents such as baking soda and baking powder is a neutralization reaction of a weak acid with a carbonate salt producing bubbles of carbon dioxide that give lightness and texture to numerous baked goods. A neutralization is a type of double replacement reaction. In a titration an acid or a base is in a flask or a beaker.













