Amazing Combustion Of Coal Balanced Equation

Hydrocarbons are chemical compounds formed by the bonding between hydrogen and carbon atoms.
Combustion of coal balanced equation. The reaction typically gives off heat and light as well. The coal fixed-bed stoker system was invented in 1822. In some cases the heat and light resulting from combustion are the pri mary goals as is the case with wood -fired cookstoves natural -gas ovens and kerosene lamps.
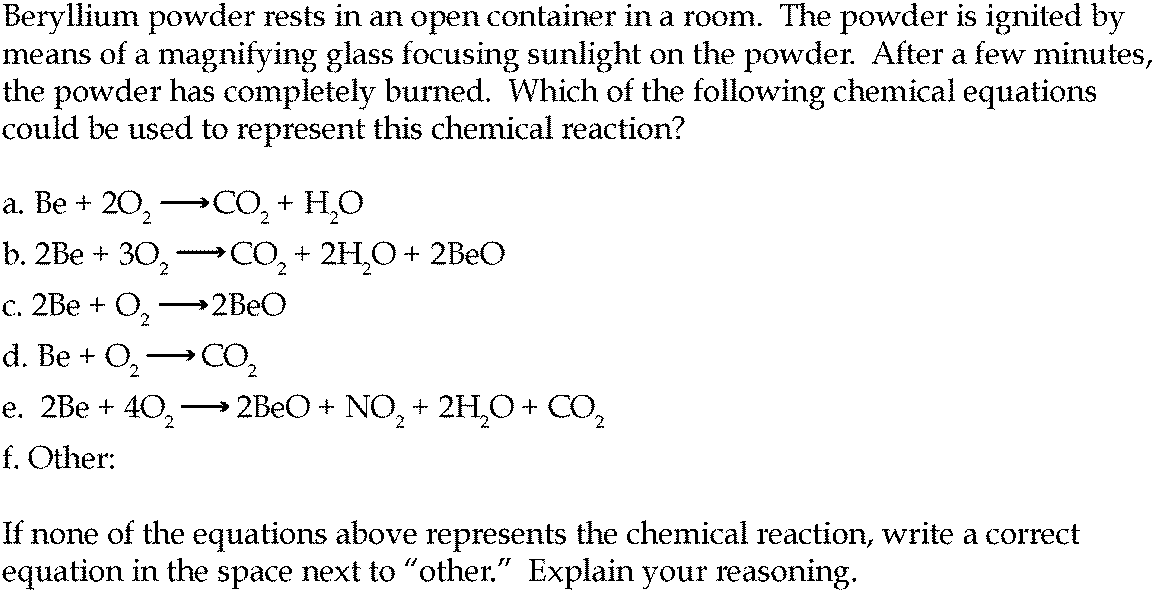
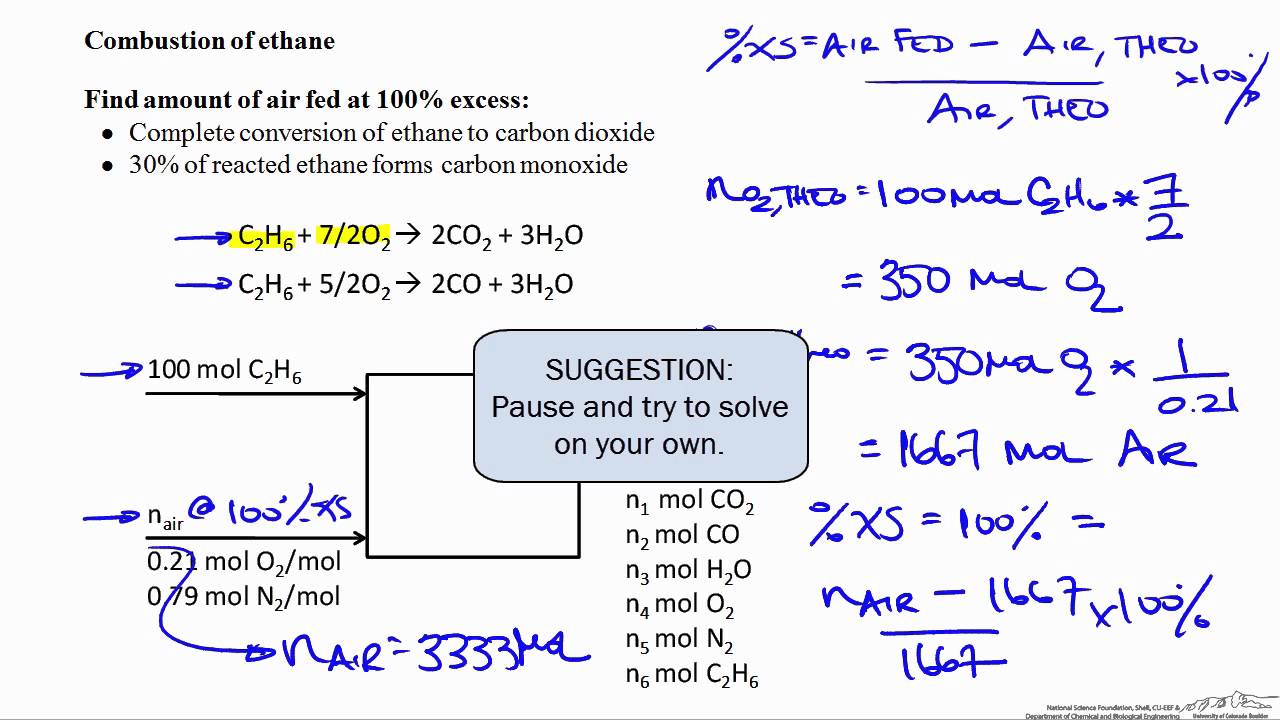
The Process of Combustion Every organic compound can be burnt in air to produce heat water vapour and carbon dioxide. The general equation for a complete combustion reaction is. Incomplete combustionIncomplete combustion occurs when the supply of air or oxygen is poor.
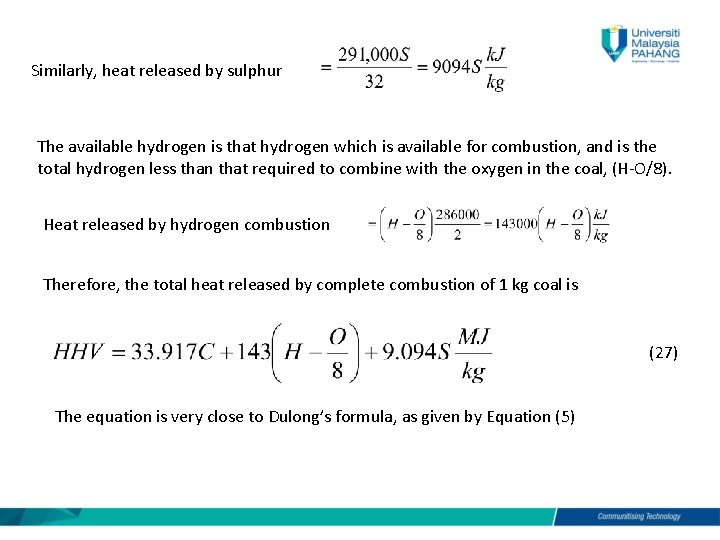
The balanced equation for the combustion of methane shows that one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water. Combustion of Coal Using Thermo Utilities MS Excel Add-ins Dry anthracite with the following composition by mass. The fuel elements in coal as in all vegetable matter are carbon and hydrogen and in the combustion process these fuels combine with oxygen from air.
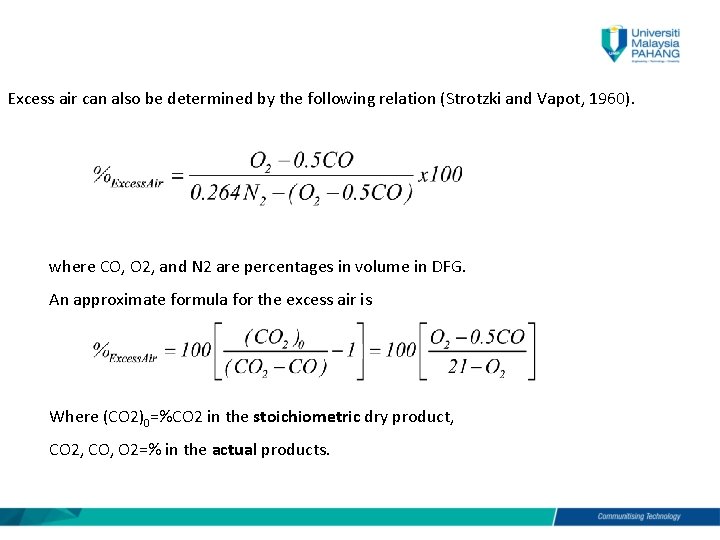
Oxy-fuel combustion is a pulverized coal combustion process practiced in coal-fired power plant. Coal rank depends on volatile. By weight the requirement of air for providing this much O 2 is After combustion of one gm carbonC product of combustion contains only 233 gm of CO and of N 2.
Fuel can be coal wood gasoline. Similarly what is Sulphur dioxide formula. The following equation is an example of.
The equa- tion can be simplified by writing 2O2 instead of O2 O2 and 2H2O instead of H2O H2O. When this equation is balanced you get. These are all combustion reactions with organic compounds.