Matchless Incomplete Combustion Equation Example

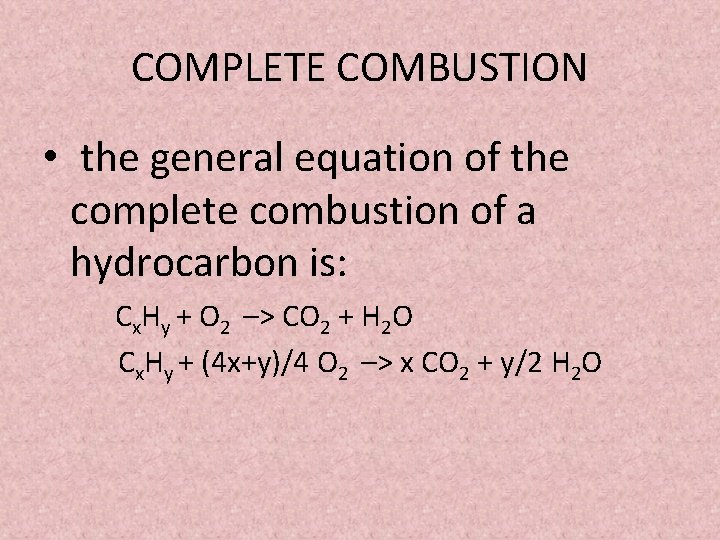
Complete combustion would be represented by the equation.
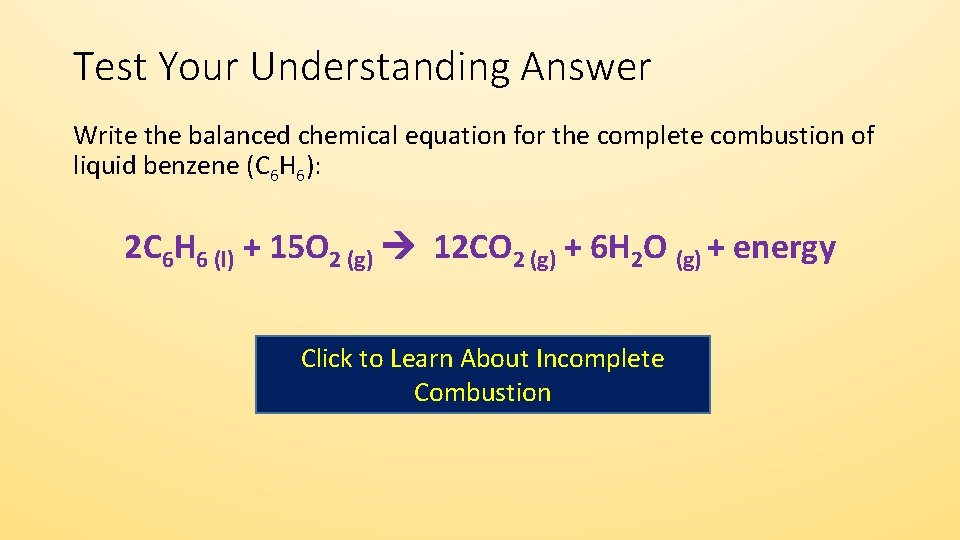
Incomplete combustion equation example. We shall take methane as an example. 2 C6H6 l 15 O2 g -- 12 CO2 g 6 H2O g Calculate the percent yield of the reaction if the combustion of 136 g C6H6 MW 7812 gmol with excess O2 MW 3200 gmol produced 401 g CO2 MW 4401 gmol 5 years ago. Some are easier than others.
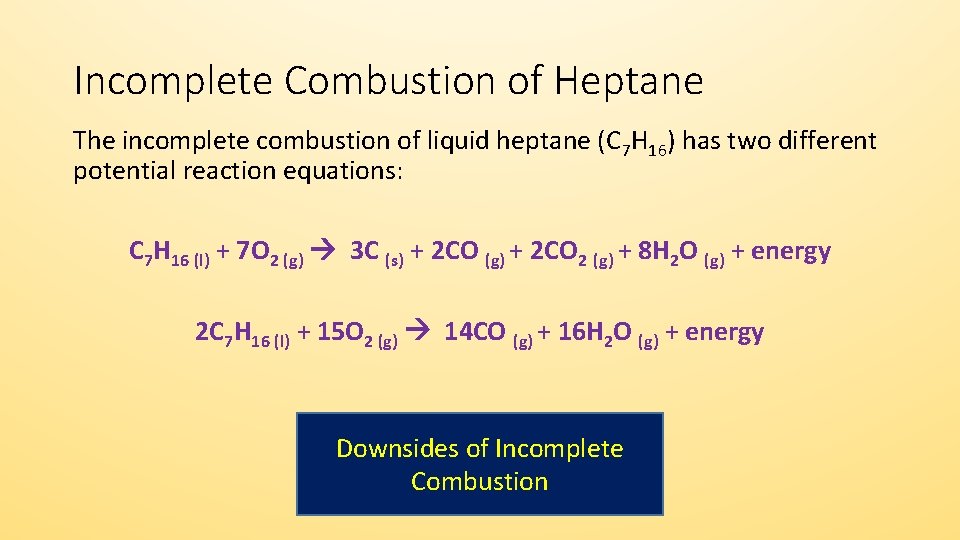
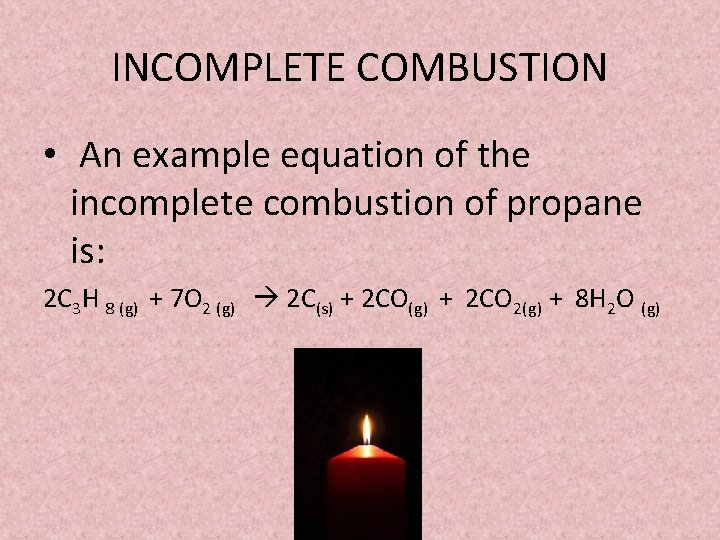
See complete combustion then some or all of the carbon turns to carbon monoxide. Water is still produced but carbon monoxide and carbon are produced instead of carbon dioxide. A Incomplete Combustion Equation - Multiple carbon products Hydrocarbon Oxygen Carbon Carbon monoxide water.
This happens with any hydrocarbon. Examples are methane CH 4 propane C 3 H 8 butane C 4 H 10 and octane C 8 H 18 Hydrocarbons readily burn or undergo combustion reactions. Hydrocarbons are compounds that contain the elements hydrogen and carbon.
The carbon is released as soot. The result of incomplete combustion is once again water vapour carbon dioxide and heat. If not enough oxygen is present for complete combustion incomplete combustion occurs.
The complete combustion of hydrocarbons leads to carbon dioxide and. Carbon monoxide is absorbed in the lungs and. For example with alkanes the ones with an even number.
For example incomplete combustion of ethylene can result in carbon and water as byproducts. Complete combustion given sufficient oxygen of any hydrocarbon produces carbon dioxide and water. An equation is equalization.