Great Hcl Naoh Balanced Equation

Balanced chemical equations are those that have an equal number of atoms of each element on both sides of the equation.
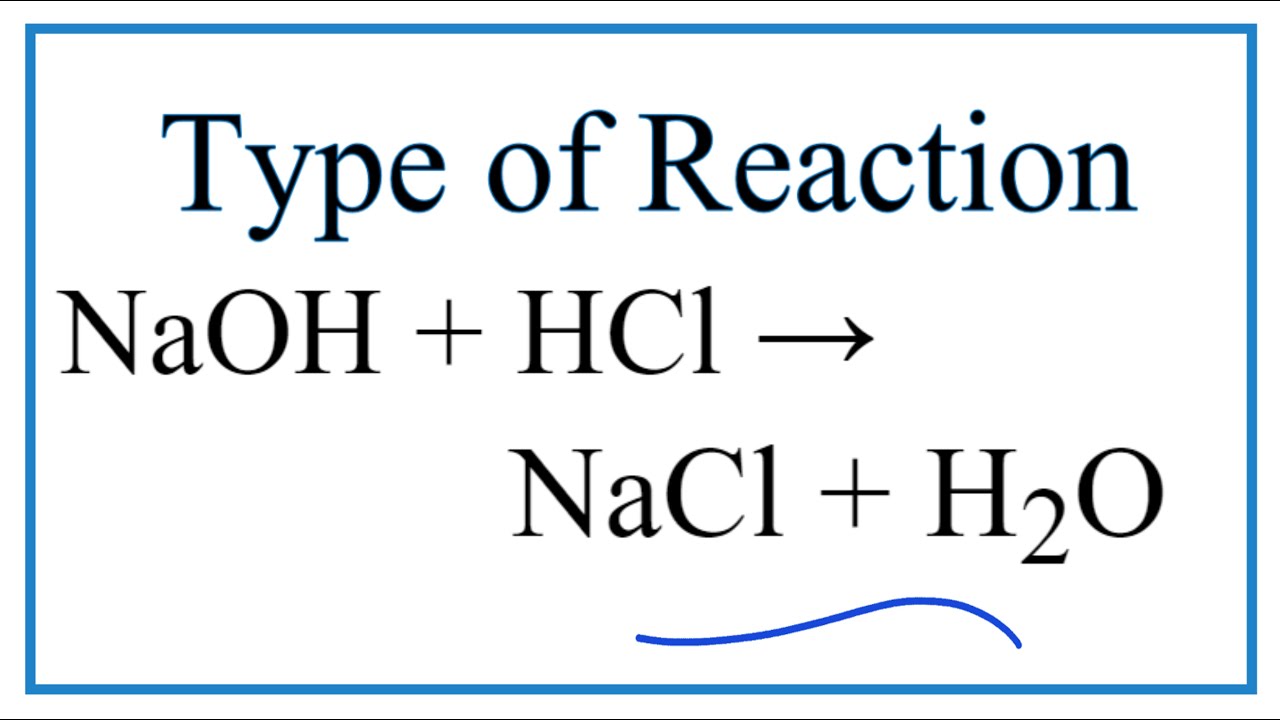
Hcl naoh balanced equation. For instance in the equation HCl NaOH NaCL H2O the HCl hydrochloric acid a strong acid and NaOH sodium hydroxide a strong base are the reactants. Fe Cl 2 FeCl 3. To write the net ionic equation for HCl NaOH NaCl H2O Hydrochloric acid Sodium hydroxide we follow main three steps.
Hydrochloric acidHClreacts with Sodium HydroxideNaOHto form a colourless aqueous solution of SodiumChloride NaCl salt. H₂SO₄ NaOH -----Na₂SO₄ H₂O Step-2In the left side we have H SO₄ Na O To balance this reaction means we need to equalize the number of these above atoms and polyatomic ion. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents.
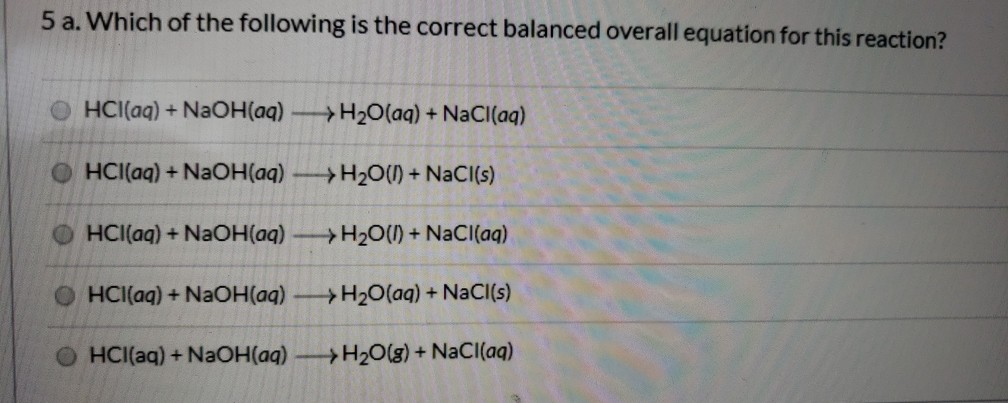
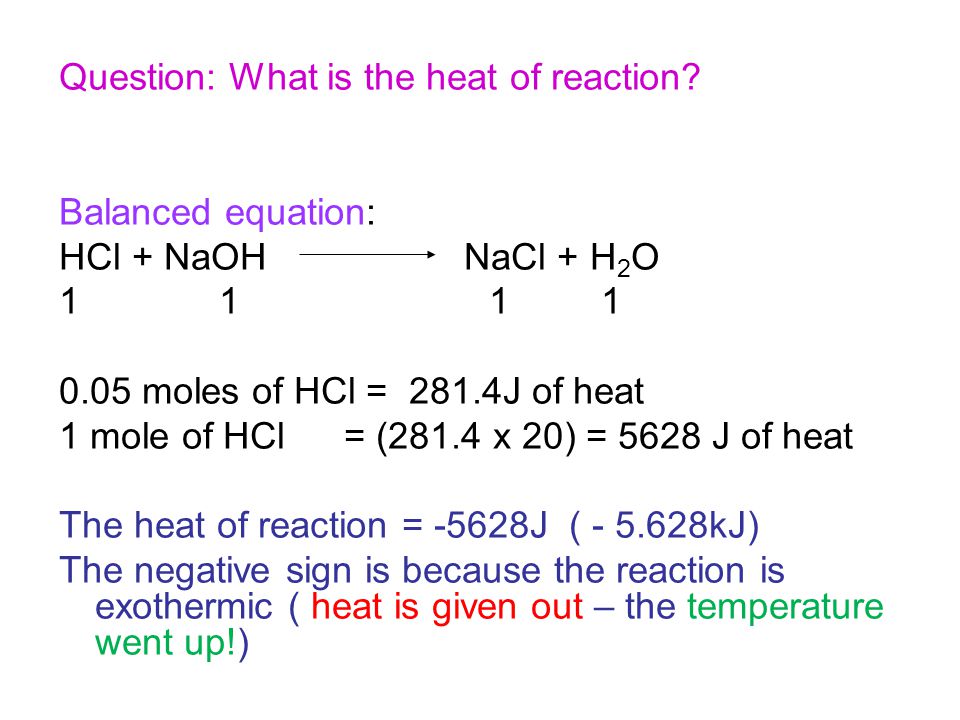
NaOH HCl -. AHneut -562 kJmol a Write a balanced chemical equation for this neutralization reaction. HCl NaOH NaCL H 2 O In ordinary synthesis reactions the final product would be the combination of reactants taking part in the chemical reaction such as follows 2Al 3Cl 2 2AlCl 3 In simple displacement reactions one part of a compounds gets replace by the other part such as follows.
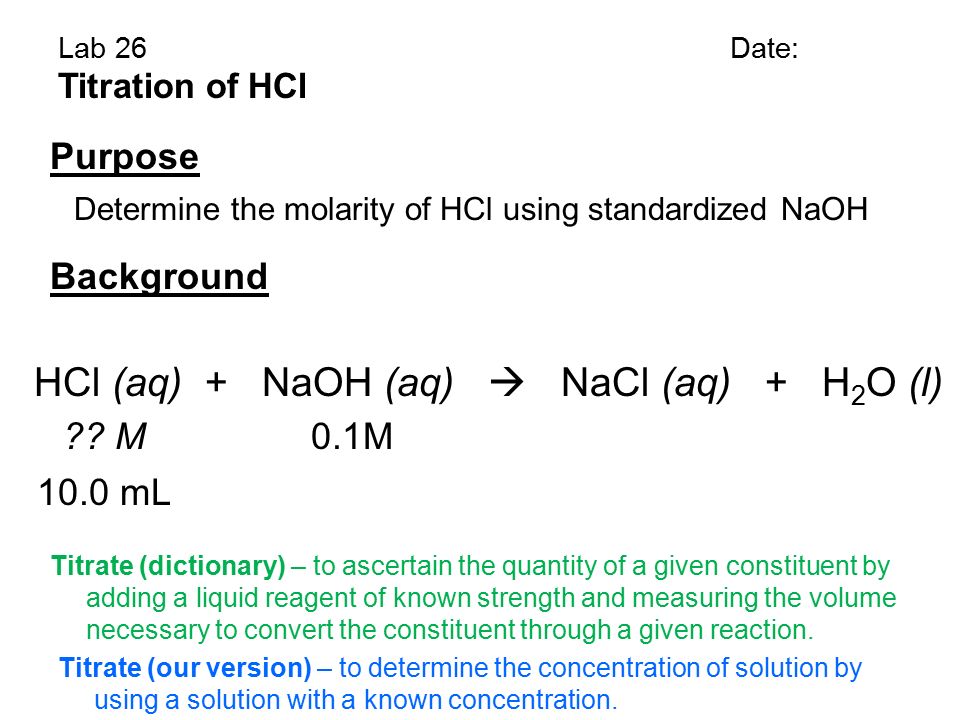
11 grams HCl 1 mole HCl36458 grams 03017 moles HCl Moles HCl same as moles NaOH Molarity moles of solute. Examples of complete chemical equations to balance. The heat exchanged by the reaction qreaction can be used to determine the change in enthalpy of the reaction.
This means that we will split them apart in the net ionic equation. What is the balanced equation when hydrochloric acid reacts with sodium hydroxide. How do you balance HCl and NaOH.
Balanced Chemical Equation 2NaOH 2HCl 2NaCl H2O HOH. HCl aq NaOH aq NaCl aq H2O l heat Calculate the number of moles of base you add to determine the molar heat of neutralization expressed using the equation ΔH Q n where n is the number of moles. In this equation they react to form the products NaCL sodium chloride or salt and H2O water.