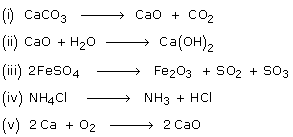Amazing Endothermic Reaction Formula Examples

Converting frost to water vapor melting boiling and evaporation in general are endothermic processes.
Endothermic reaction formula examples. A constant input of energy often in the form of heat is needed to keep an endothermic reaction going. An exothermic reaction is a reaction in which energy is released in the form of light or heat. One of the most important series of endothermic reactions is photosynthesis.
The equation for this reaction is. Photosynthesis is an example of an endothermic chemical reaction. In an exothermic reaction change in enthalpy.
6CO2 6 H2O heat --- C6H12O6 6O2. Heat location is the quickest way to find if a reaction is endothermic or exothermic. Reactants Energy Products.
The general equation for an exothermic reaction is. An exothermic reaction is a chemical reaction that releases heat and has a negative enthalpy -ΔH and positive entropy ΔS. Common processes and solved examples.
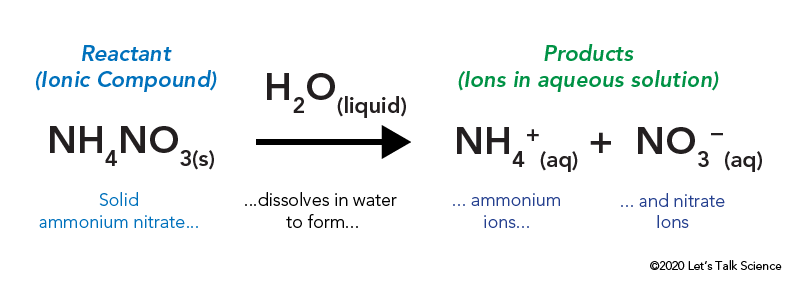
Melting ice is an example of this type of reaction. There are many possible ingredients in an instant cold pack but they often contain solid ammonium nitrate and water. Energy Change in Endothermic Reactions The general equation for an endothermic reaction is.
These reactions are energetically favorable and often occur spontaneously but sometimes you need a little extra energy to get them started. As the heat is absorbed the product will be colder. The reaction equationNH4NO3 s water NH4 aq NO3 aqThis is an example of endothermic reaction because the temperature drops because heat energy is taken in by the reaction mixture.