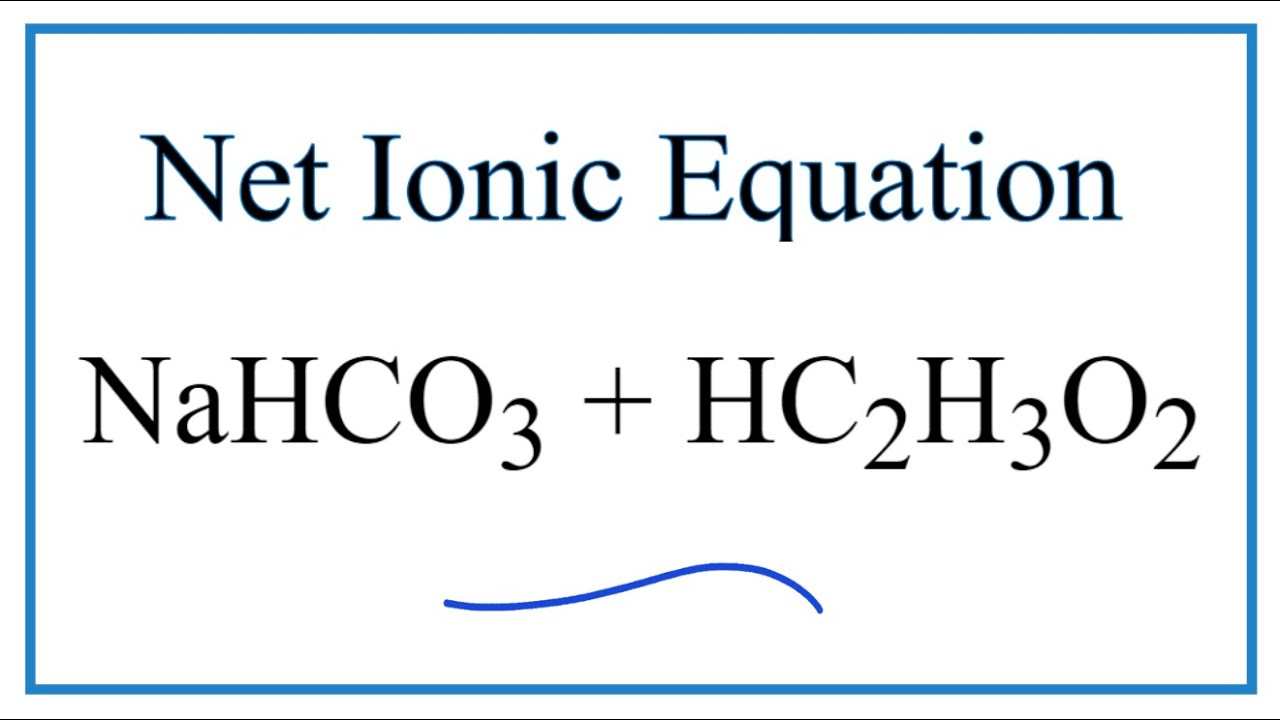Sensational Balanced Chemical Equation Of Vinegar And Baking Soda

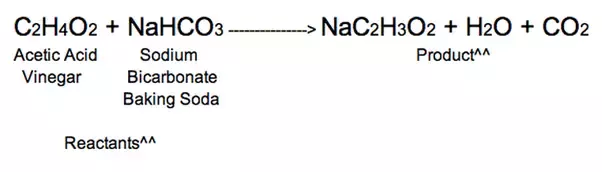
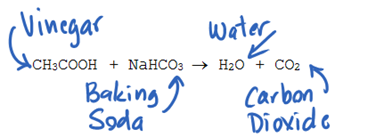
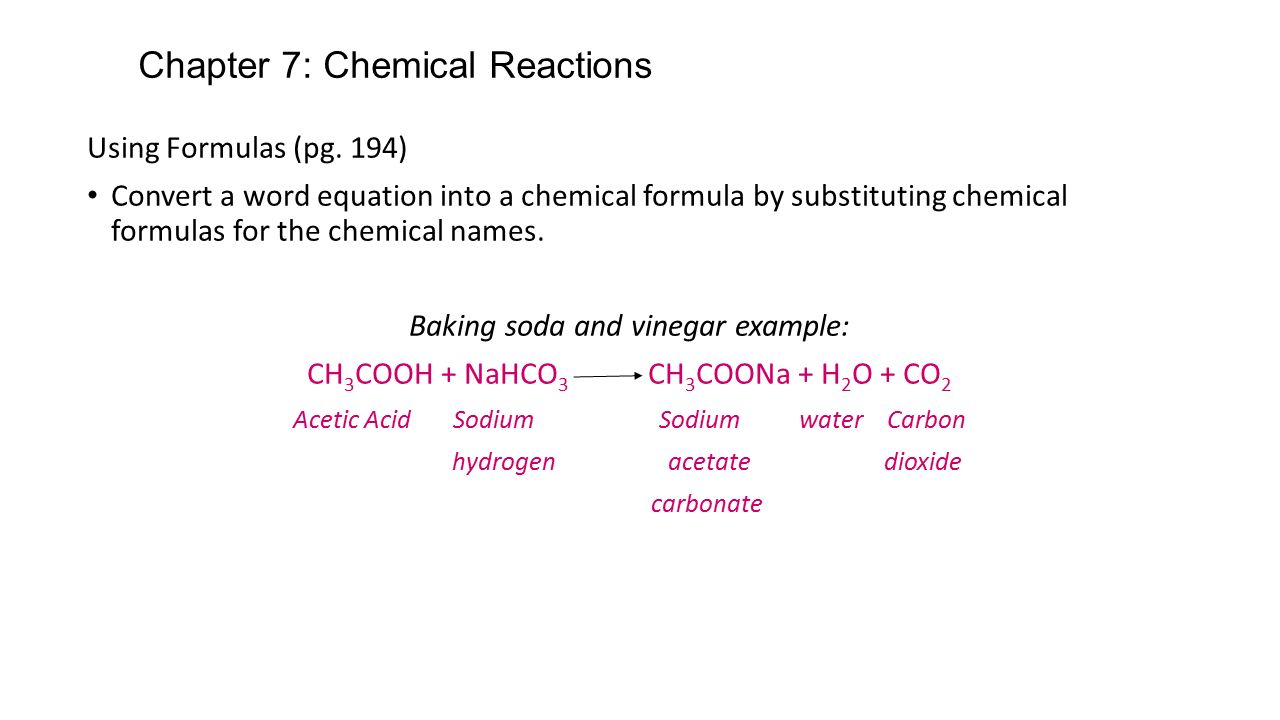
CH3COOH NaHCO3 CH3COONa CO2 H2O.
Balanced chemical equation of vinegar and baking soda. It explains how to write the net ionic equation of the reaction betwee. This equation is already balanced in nature. Repeat for all three balloons 9.
There is a discussion about how there is a classic equation and a more technically correct equation here. For this chemical equation to balance the number of hydrogen ions that supplies shall be the same as the number of these ions that consumes. Each bicarbonate ion will consume one hydrogen ion to produce water and carbon dioxide.
It is backing soda and vinegar and it forms sodium acetate CO2 and a substance that turns blue cobalt paper pink. Write the balanced chemical equation for the reaction of baking soda with vinegar see og 1 above Chemistry 10 2020-2021 online Page 1 Properties of Gases C2. Knowing this we can assume that the mole ratio is 11.
Acetic acid present in vinegar will readily react with baking soda sodium bicarbonate to form sodium acetate with the effervescence of carbon dioxide. Vinegar is acetic acid C 2 H 4 O 2 and water. Apparently there is more to this than I had originally thought.
NaHCO3 s _HC2H302 1 _NaC2H302 aq _H2CO3 aq 12. The end products are carbon dioxide a gas and water in which two chemical products sodium ions and acetate ions are dissolved. Vinegar - A dilute solution of acetic acid in water.
To calculate the moles of sodium hydrogen carbonate we needed to to get the molar mass of the. NaHCO3 aq CH3COOH aq ---. Each formula unit of contains one bicarbonate ion.