Simple Propane Oxygen Reaction

Propane oxygen water carbon dioxide Once you can write word equations practice by writing the formula underneath 2.
Propane oxygen reaction. What volume of carbon dioxide id produced when 28 L of oxygen are consumed. Fuels are substances that react with oxygen to release useful energy which consists of mostly heat and light. Propane C3H8 reacts with oxygen in the air to produce carbon dioxide and water.
The torch head is gaffed to the table top with enough slack to turn the gas valves. When a hydrocarbon such as propane burns in oxygen the products of this reaction is always water and carbon dioxide if complete combustion occurs which requires a good supply of oxygen or air. Propane releases its chemical energy by undergoing hydrocarbon combustion.
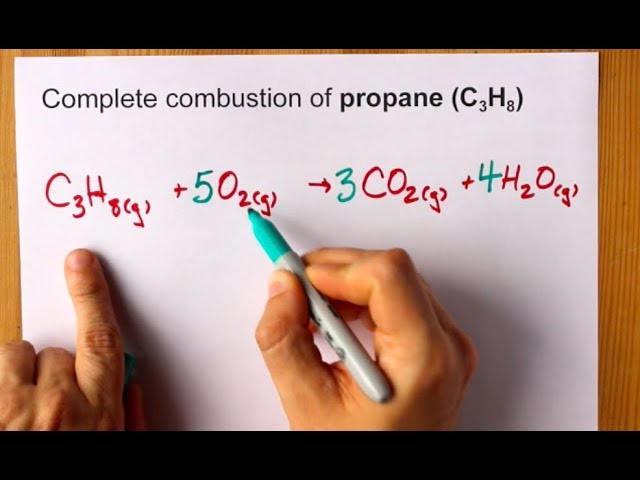
Oxidation of propane to acrolein and ammoxidafion to acrylonitrile with molecular oxygen proceed over complex metal oxide catalysts. Chemical Reactions A chemical reaction is a process that produces a chemical change to one or more substances. C 3 H 8 5O 2 3CO 2 4H 2 O Heat Energy.
Propane C3H8 burns in oxygen to produce carbon dioxide gas and water vapor. In a particular experiment 380 grams of carbon dioxide are produced from the reaction of 2205 grams of propane. Complete combustion of a hydrocarbon fuel happens when there is a good supply of air.
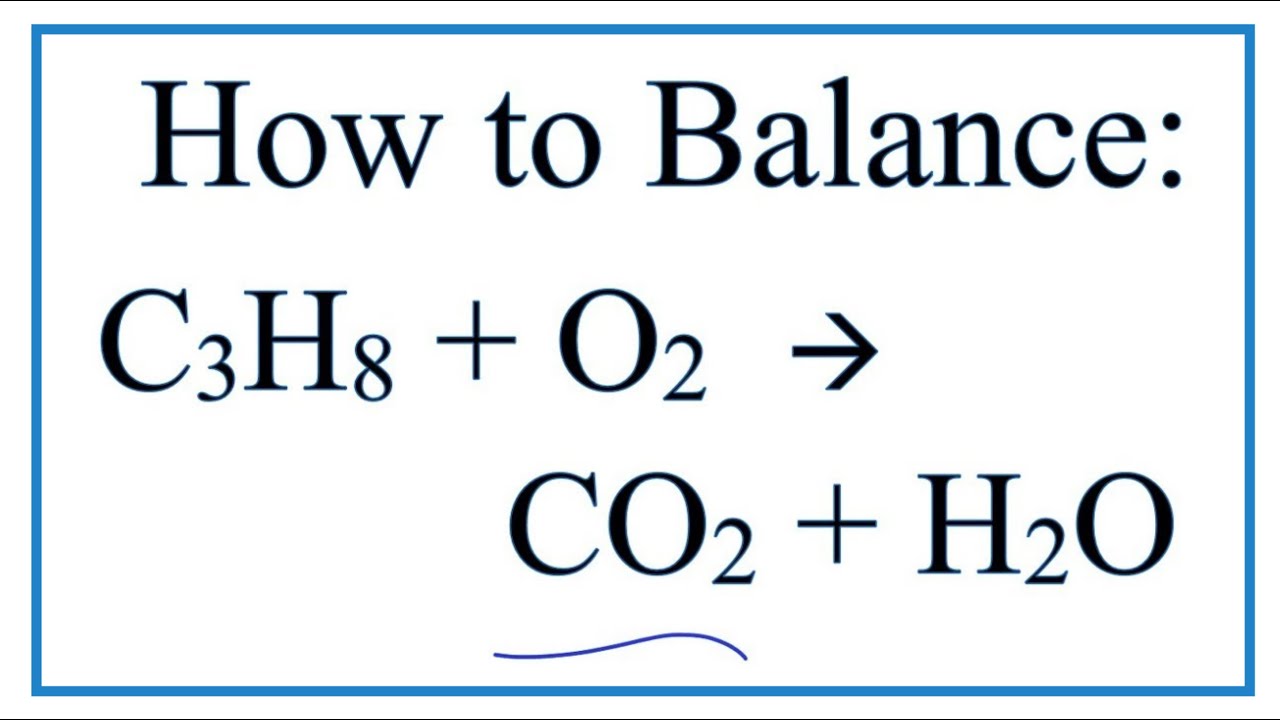
Propane C3H8 burns in oxygen to produce carbon dioxide and water vapor. Below is a hydrocarbon combustion animation showing the net reaction that occurs when propane combines with oxygen. The balance equation for this reaction is C3H8 5O2 ---- 4H20 3CO2.
Carbon dioxide and water are. Propane is a flammable naturally occurring hydrocarbon gas that is able to react with oxygen to be used as a fuel. To balance an equation first write down the.