Smart Khp Naoh Titration Calculations

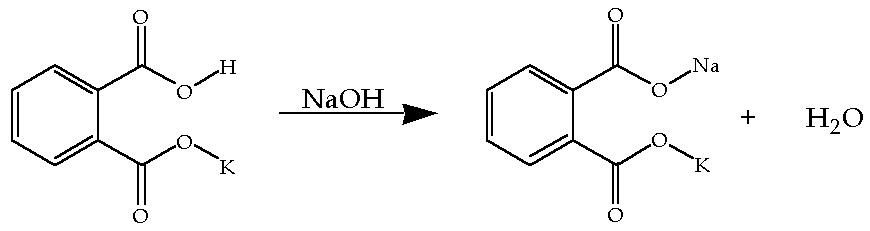
Convert grams of KHP to moles of NaOH using the balanced equation for the reaction of KHP with NaOH.
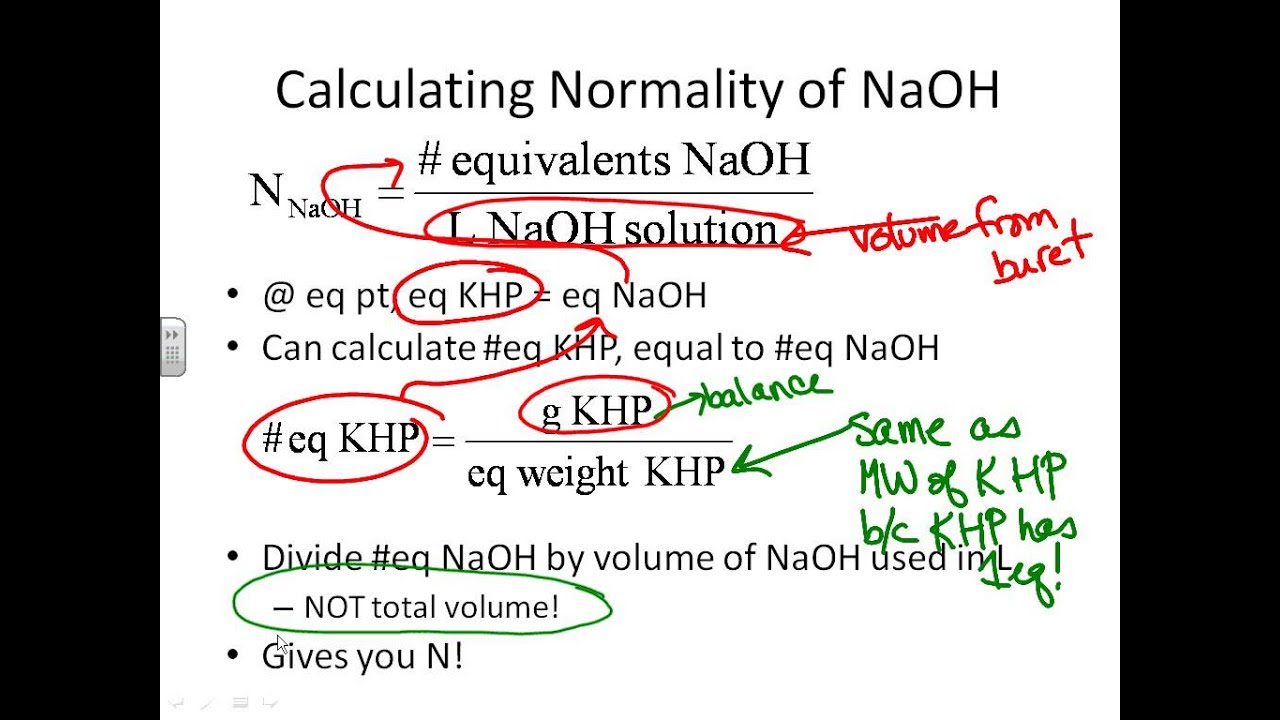
Khp naoh titration calculations. V L Moles NaOH M NaOH NaOH 2. Use your average value of NaOH volume to calculate its molarity. Calculate the percentage KHP in the unknown sample mass of 09735 g.
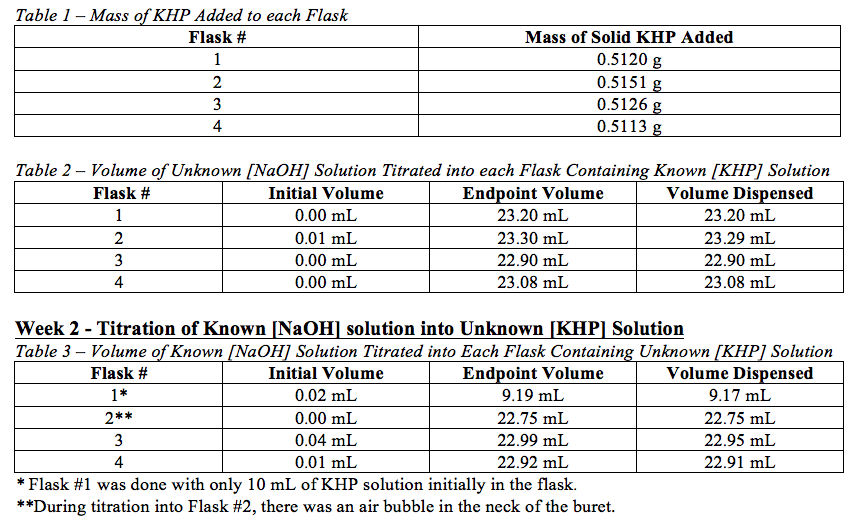
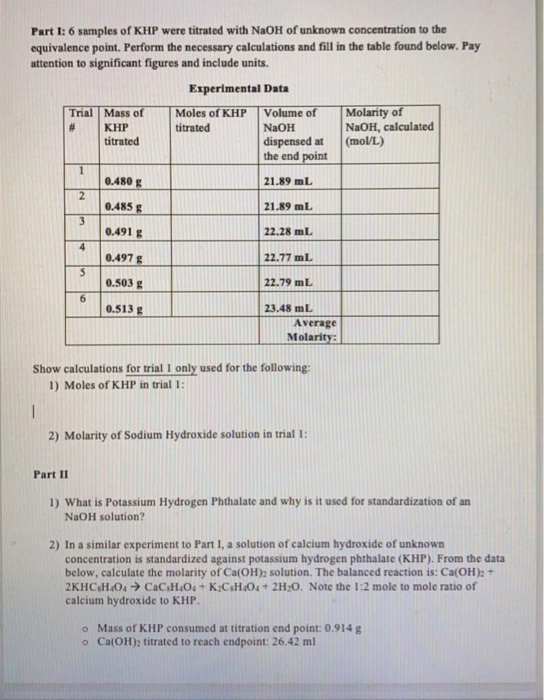
TITRATION PROCEDURE You are to determine the true molarity of the NaOH solution to four significant figures by titration of a known mass of KHP as specified on the drill sheet. 009889M Trial i Trial 2 Trial 3 Volume of standardized NaOH solution used 480 ml 470 mL 470 mL Mass of unknown KHP sample 17528 16889 1733 by mass KHP sample Average mm Standard deviation Show your calculations. Determination of the Unknown Acid Concentration Example.
Calculate the moles of KHP NaOH and the molarity of the NaOH. Trial 1 Trial 2 Trial 3 Mass or KHP 03130 1672mL 120mL 03046 3180-L 03059 1513mL Final Buret Reading Initial Buret Reading 1672mL 000ML Calculations. Mass of KHP g Volume of NaOH used in titration ml To be determined.
Accidentally overshoot the endpoint weigh a fresh sample of KHP and repeat the titration. Calculate the formality of the NaOH for each titration and find the average formality. Moles of KHP Moles of NaOH Known.
01000 molL NaOH x 002500 L NaOH x 1 mol KHP1 mol NaOH 00025 mol KHP. At the equivalence point. All you need to do is multiply the number of each type of atom by its atomic mass which you can find on the back cover of the lab manual and then add up the total masses for each element.
You cant use the unknown KHP to find the concentration of NaOH as this assumes the KHP is 100 or close to it. Moles NaOH Moles KHP 3. Repeat steps 1 to 4 twice to obtain a total of 3 trials.