Out Of This World Iron Nail Chemical Formula
The rusting of iron is actually a good example of the process of corrosion.
Iron nail chemical formula. It is the second most common metal in the Earths crust after aluminum. Iron nail Iron nail. Iron ˈ aɪ ər n is a chemical element with symbol Fe from Latin.
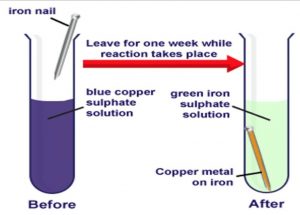
The equation for the process is. The overall chemical equation for the formation of rust is. This reaction is called displacement reaction in which the reactive element Iron Fe has displaced less reactive Copper from its solution The chemical equation can be written as.
O 2 aq 2H 2 O aq 4e 4OH The Fe 2 ions then chemically react and bond with the OH ions in water to create iron hydroxide which eventually dries up to form rust. The acidity within the removed protective coating facilitates. It is by mass the most common element on Earth right in front of oxygen 321 and 301 respectively forming much of.
Iron III Hydroxide Fe 2aq 2OH aq Fe OH 2 aq Fe 2 O 3 s rust. Under wet conditions iron will rust more quickly. Iron III oxide or ferric oxide where the iron atom exhibits an oxidation state of 3.
Iron water oxygen rust. Wikipedia page Fe iron is a silver-white metal with a wide range of applications. In its pure form iron is a soft malleable substance that rusts easily when exposed to air and moisture.
The iron is gradually eaten away as it reacts slowly with the oxygen. Painting your nails with nail polish might not seem like a particularly complex chemical process but theres much more to it than meets the eye. 4 Fes 6 H 2 Ol 3 O 2 g 4 FeOH 3 s IronIII hydroxide FeOH 3 then dehydrates to produce Fe 2 O 3nH 2 Os or rust.