Neat General Equation For Exothermic Reaction
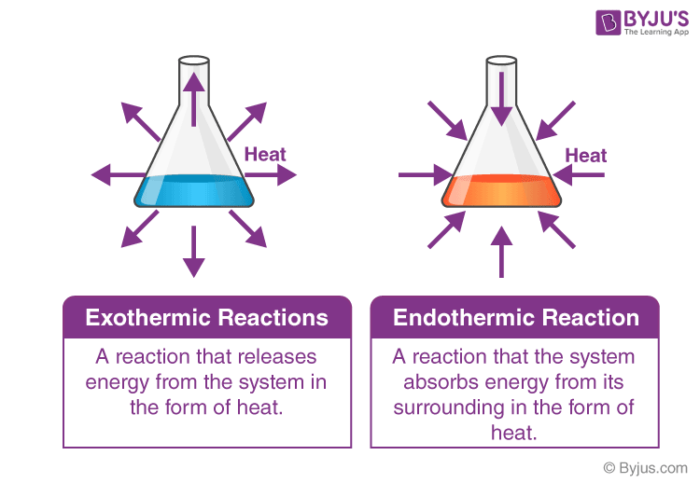
This classification of chemical reactions takes into account the participation of energy either as a reactant or as a product.
General equation for exothermic reaction. Respiration is another exothermic reaction. Copypaste the general equation for an exothermic reaction Reactants Products from CHEMISTRY INORGANIC at Northwest Career And Technical Academy. And this reaction releases energy.
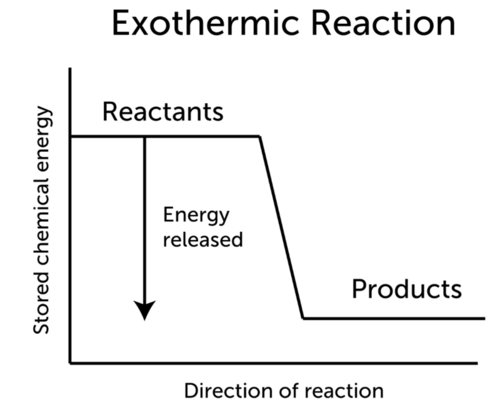
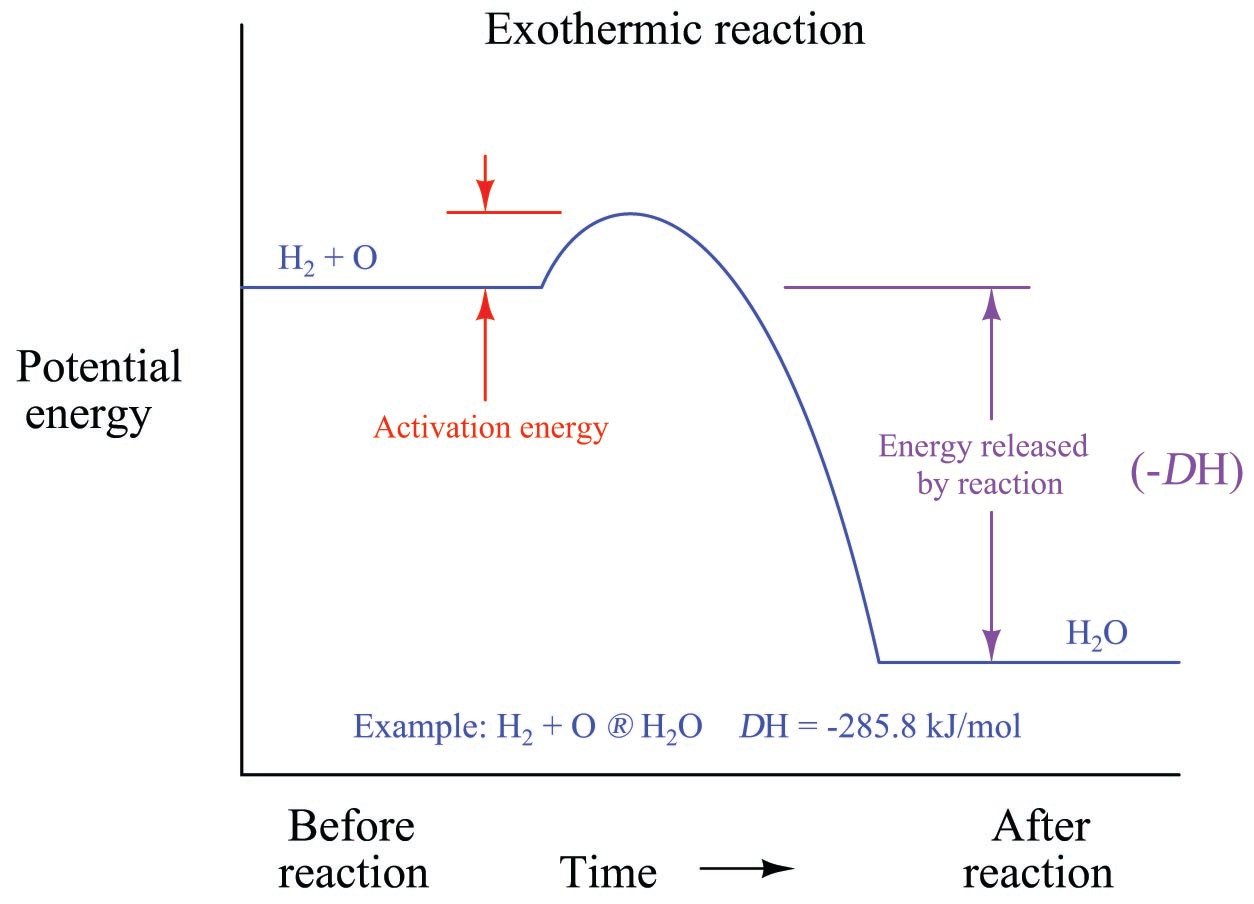
This video relates to the AQA 9-1 GCSE Chemistry specification which will be examined for the first time in 2018. If the energy is released as heat an exothermic reaction results in a rise in temperature. ΔH represents the change in energy.
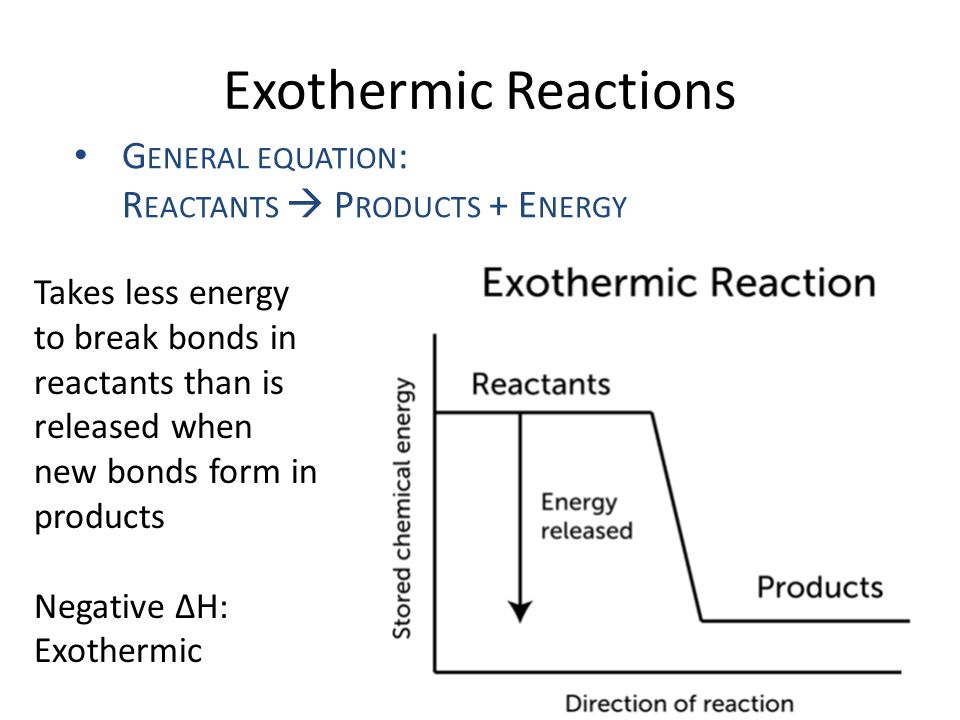
The general equation for an exothermic reaction is. If the energy produced in an exothermic reaction is released as heat it results in a rise in temperature. 7311 CaCO 3 s CaO s CO 2 g Δ H 1778 kJ Exothermic Reaction.
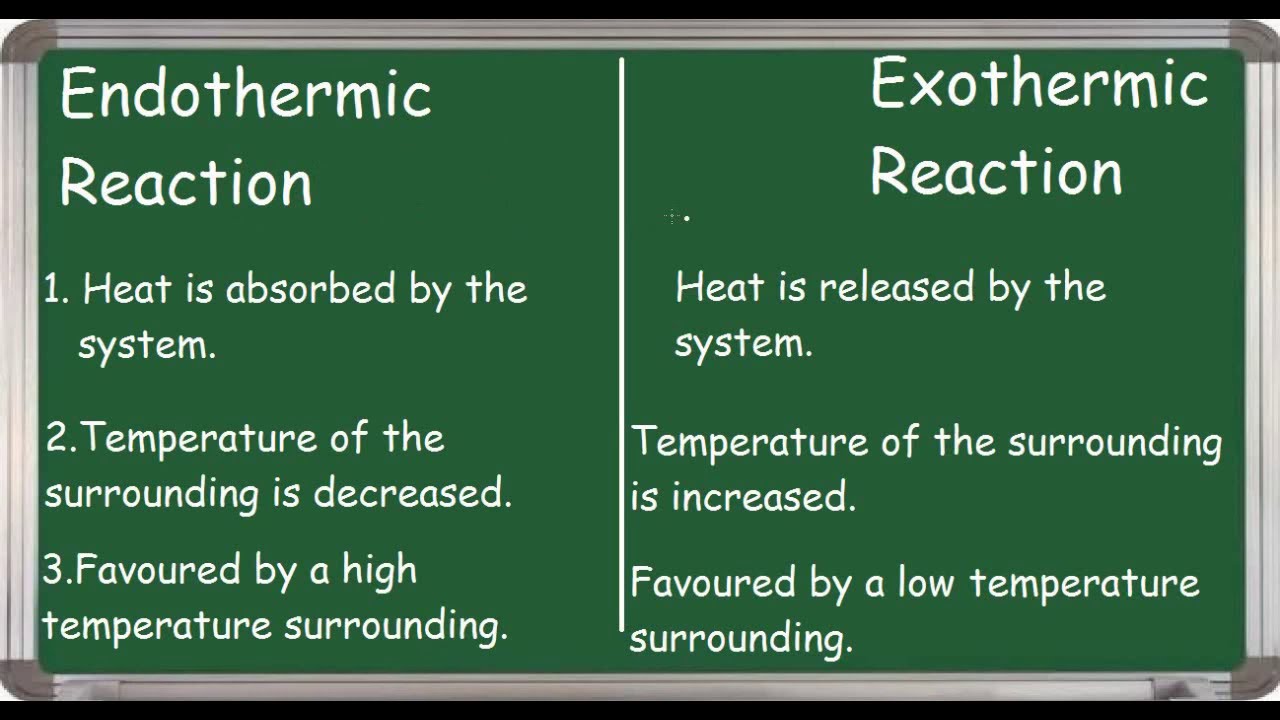
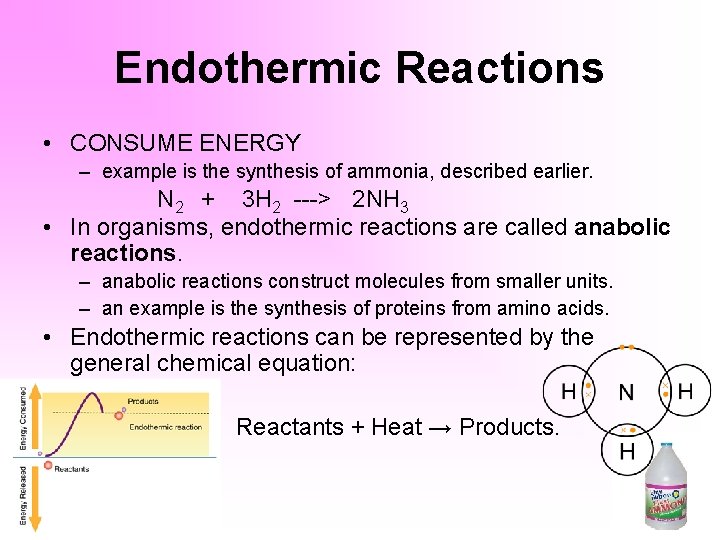
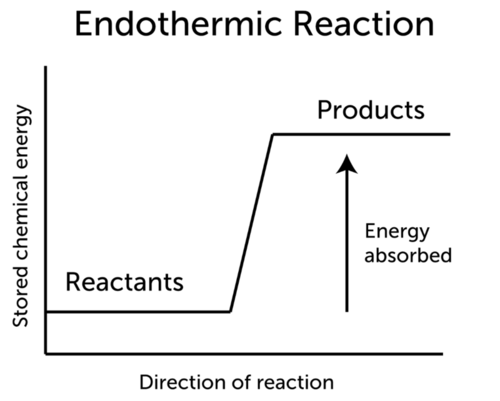
An endothermic reaction requires energy while an exothermic reaction releases energy. Reactants Products Energy. Reactants Products Energy.
As a result the products are likely to be warmer than the reactants. The general equation for an exothermic reaction is. Is respiration exo or endothermic.
The general equation for an exothermic reaction is. The reduction of iron III oxide by aluminium releases sufficient heat to yield molten iron. Thats what happens in the exothermic reaction at the URL below.

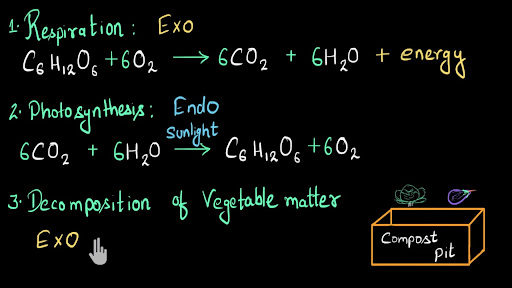



/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)