Sensational Conditions Needed For Iron To Rust

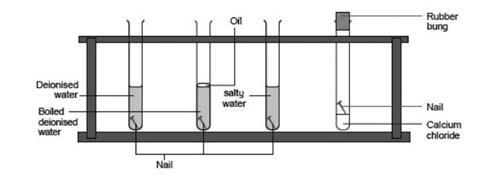
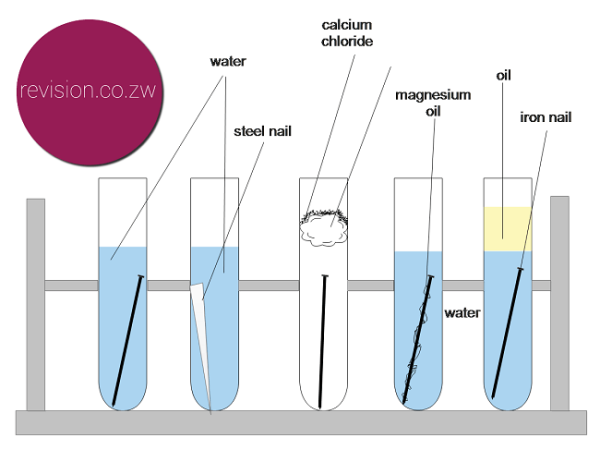
Iron is susceptible to rusting Rusting can only occur in the presence of moisture water and air Controlling humidity test tube C prevents rusting Galvanising using a more reactive metal such as zinc prevents the rusting of iron.
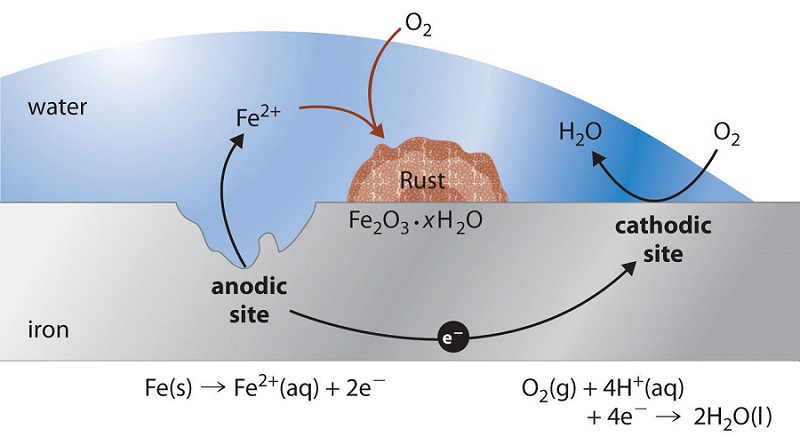
Conditions needed for iron to rust. They are formed by the chemical reaction between iron and oxygen in the presence of water. Rusting is an oxidation reaction. This reaction is not instantaneous it generally proceeds over a considerably large time frame.
Here is the word equation for the reaction. Rusting is an oxidation reaction. Rust is what we call the oxidation of common metals usually specifically iron or steel.
Things like heat moisture catalysts salts acids bases etc. This active metal loses electrons undergoes oxidation in preference to iron and hence prevents the rusting of iron. Conditions Necessary For Rusting Rust or iron oxide is a pretty common compound and it usually appears as a reddish coating on iron or steel.
For oxidation you just need oxygen. Each boiling tube holds a nail in water salty water water with oil on top or just oil. Rust forms when oxygen reacts with iron but simply putting iron and oxygen together isnt sufficient.
Iron water oxygen hydrated iron III oxide Iron and. They can then determine what conditions are required in order for iron to rust. 1 See answer aleerajanellsunico aleerajanellsunico Answer.
Rust is a general term for various forms of iron oxide. Aluminium on the other hand does not corrode easily because its surface is protected by a layer of aluminium. Conditions for Rusting Iron and steel rust when they come into contact with water and oxygen.