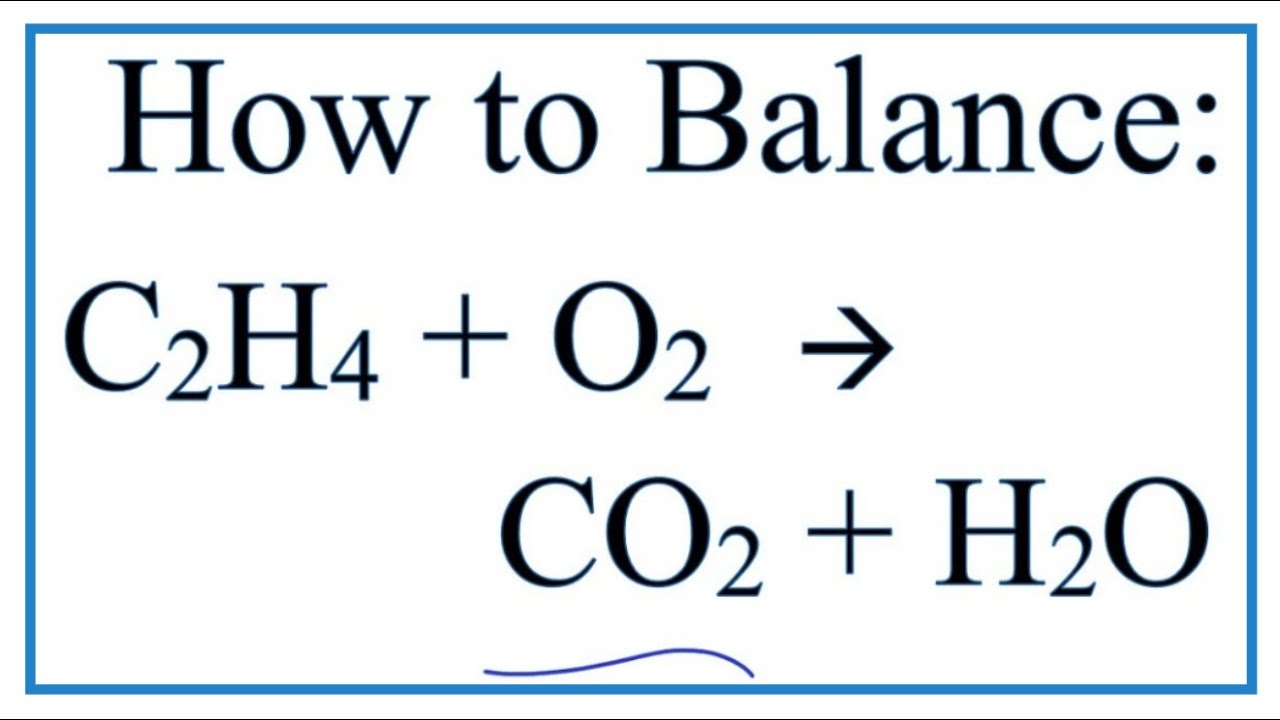
Favorite Balanced Ethane Combustion Equation
In order to balance C2H6 O2 CO2 H2O youll need to watch out for two things.
Balanced ethane combustion equation. Ethane C_2H_6 reacts with molecular oxygen to produce carbon dioxide and water. Click hereto get an answer to your question The balance equation for the complete combustion of ethane is. In the reaction the bonds in the methane and oxygen come apart the atoms rearrange and then re-bond to form water and carbon dioxide.
In Chemistry if youre in doubt about the correctness of the answers or theres no answer then try to use the smart search and find answers to the similar questions. The balance equation for the complete combustion of ethane. During the combustion of 500 g of ethane C2H6 355 kcal is released a Write a balanced equation for the combustion of ethane b What is the sign of H for this reaction.
2 C 2 H 6 7 O 2 4 CO 2 6 H 2 O Heat Energy Enthalpy The hydrocarbon combustion reaction releases heat energy and is an example of an exothermic reaction. The balanced chemical equation for the complete combustion of ethane is. Consider the combustion of ethane.
The correct balanced equation for incomplete combustion of ethane will be Explanation. Write and balance the equation for the complete combustion of ethane C2H6. A combustion reaction has a general reaction of.
2C_2H_67O_2 - 4O_26H_2O In a combustion reaction with a hydrocarbon in the reactant side you will always have O_2 as another reactant. Because the question asks for complete combustion the products are water H2O and carbon dioxide CO2. Write a balanced equation for the combustion of Methane.
Ethane is an alkane with the chemical formula C2H6. What is the balanced equation for ethane. Combustion is a type of reaction that involves combustible material and an oxidizer to form an oxidized reaction so it.