Simple Airbag Chemical Reaction Equation

Really its the noise of an explosion.
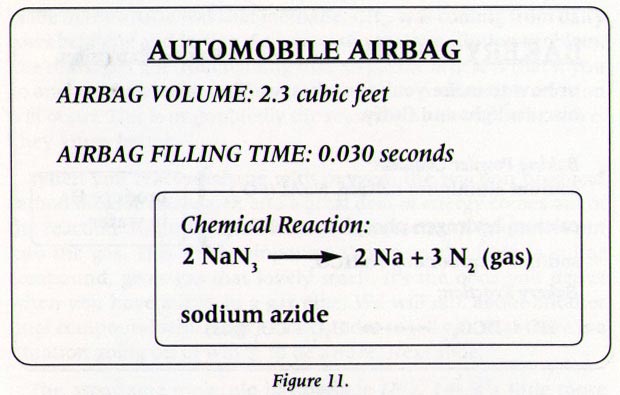
Airbag chemical reaction equation. And their chemical formulas involved in airbag deployment based on the. K20 Na20 SiO2 alkaline silicate glass. SC4a Plan and carry out an investigation to provide evidence of the effects of changing concentration temperature and pressure on chemical reactions.
Those reactions are listed above. 1Make a list of the chemicals. During an accident the following chemical reactions occur.
Obtain evaluate and communicate information about how to refine the design of a chemical system by applying engineering principles to manipulate the factors that affect a chemical reaction. Based on the chemical equation 2 NaN 3 -- 2 Na 3 N 2 a cup of the compound can easily produce enough nitrogen gas to fill a standard airbag which is close to 70 liters. What about the Gas Used to Fill the Airbag.
KNO3 K20 Na2O N2 g Final Reaction. The balanced equation will appear above. The chemical reaction produces a gas that inflates the airbag the gas that the chemical reaction produces is nitrogen gas.
Reactions Science Videos May 01 2018. When car sensors detect a crash a chemical reaction is triggered by the ignition of a. But the other product of the reaction sodium is not a benign chemical.
The sodium from the first reaction and the potassium nitrate. Fe Au Co Br C O N F. 2 NaN 3 s 2 Na s 3 N 2 g 10 Na s.